Fig. 2.
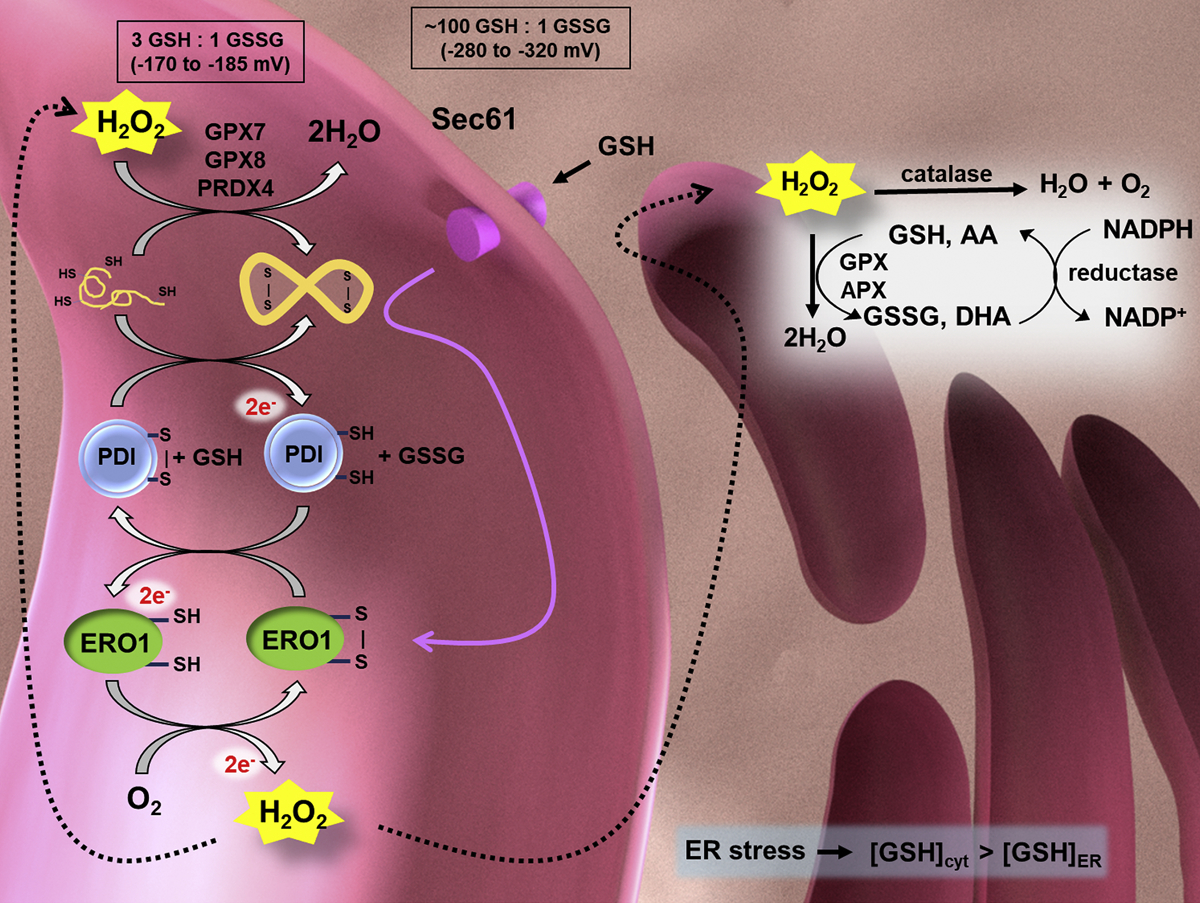
The ER (pink) is an oxidizing environment, maintained by redox sensor glutathione (GSH/GSSG), with a reduction potential much larger than that of the cytoplasm (ER ε: −170 to − 185 mV; cytoplasm ε: −280 to −320 mV). The oxidizing environment promotes nascent protein folding and disulfide bond formation. ERO1 is a key mediator of disulfide bond formation in the ER. Disturbed protein folding may cause ER stress and increased ER ROS production, further affecting the mitochondrial and cellular metabolism. Reduced polypeptides are oxidized by PDI, which transfers its electrons to ERO1. ERO1 is reoxidized by oxygen and produces H2O2. H2O2 is reduced through various mechanisms in the ER including catalase, glutathione peroxidases (GPX7 and GPX8), peroxiredoxin 4 (PRX4), and ascorbate peroxidase (APX). Background image created in Blender 2.79.
