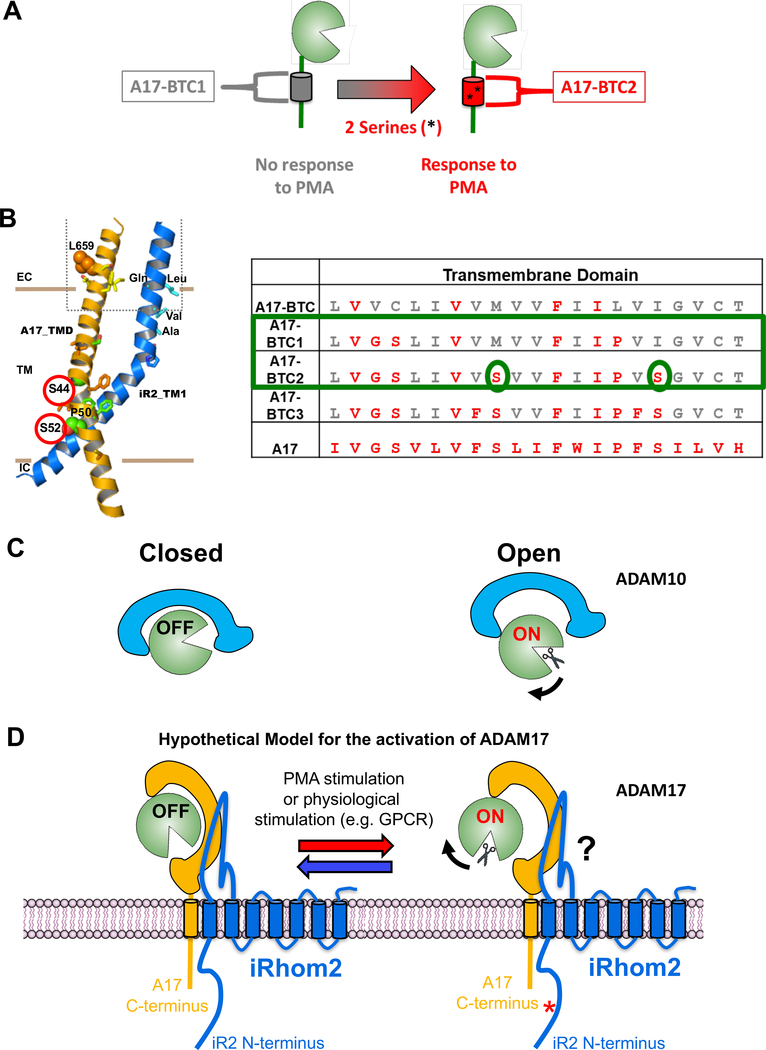
Figure 6. Diagrams indicating the predicted interactions between iRhom2 and ADAM17, the location of TMD serine residues that are required for ADAM17 activity and of a hypothetical model of the possible regulation of ADAM17 by iRhom2.
(A) Diagram of the role of Serine S44 and S52 in restoring PMA-stimulated activity to ADAM17-BTC1. (B) A Molecular Dynamics modeling of the predicted interaction between the ADAM17 TMD and the iRhom2 TMD1 (33), with S44 and S52 highlighted by red circles. The amino acid sequences of the TMD of A17-BTC, A17-BTC1 – 3 is shown for comparison, with S44 and S52 highlighted by green ovals. (C,D) Model of the possible allosteric regulation of the extracellular domain of ADAM17, based on the recent crystallographic identification of an “on” and “off” position in the highly related ADAM10 (C, adapted from (56)). We hypothesize that the interaction between the TMD/JMD of ADAM17 and the TMD1/JMD1 of iRhom2 provides a fulcrum that allows the complex to reversibly toggle between two allosteric conformations, one “on” and the second “off” (D). This switch could be activated by phosphorylation of the cytoplasmic tail of iRhom2 (34). Further studies will be necessary to understand how this switch is controlled and how it could exert allosteric control over the activation of ADAM17.