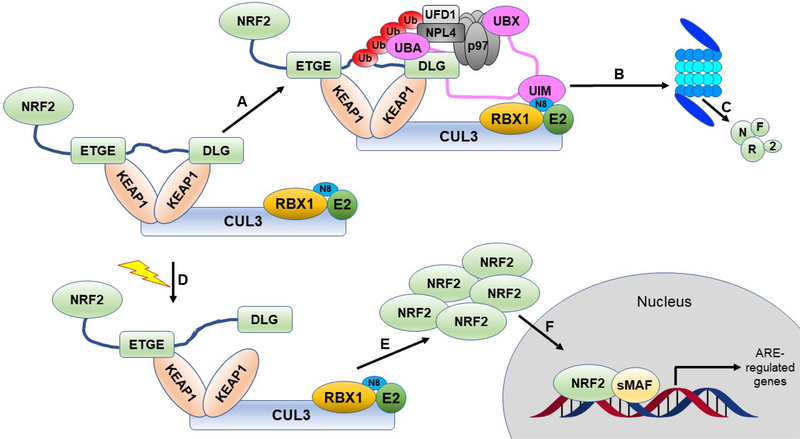
Figure 2. The canonical NRF2 pathway.
NRF2 is sequestered by the CUL3-RBX1 E3 ubiquitin ligase complex through the KEAP1 adapter protein, which binds the ETGE and DLG motifs of NRF2 in a 2:1 KEAP1:NRF2 ratio. A) Under basal, unstressed conditions, the CUL3 complex ubiquitylates NRF2 at one of the seven lysines residing between the ETGE and DLG motifs. B) Ubiquitylated NRF2 is then extracted from the CUL3 complex through the action of p97-UFD1-NPL4 mediated by UBXN7. C) Ubiquitylated NRF2 is transferred to the 26S proteasome, where it is destroyed. D) When cells are challenged with an oxidative or xenobiotic insult, one of the sensor cysteines of KEAP1 can become modified, which causes a structural rearrangement, releasing the DLG motif, and stopping subsequent ubiquitylation. Please see the text for alternate explanations and other models. E) The inhibited CUL3 complex blocks further NRF2 degradation, allowing NRF2 levels to rise in the cytosol. F) They then translocate to the nucleus, where they can bind to sMAF proteins and initiate ARE-regulated transcriptional programs.