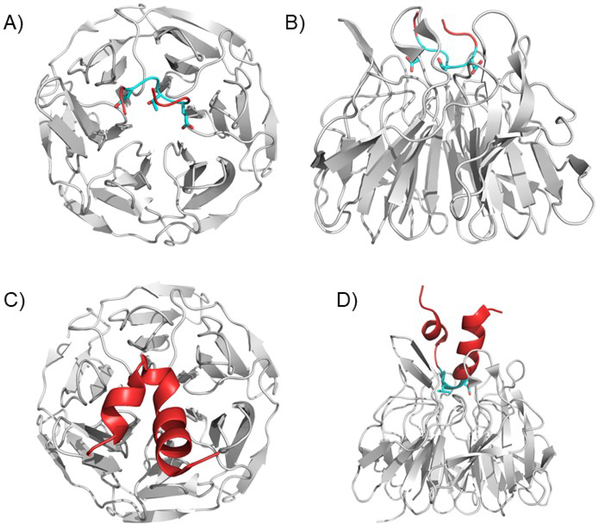
Figure 4. The KEAP1 Kelch domain bound to ETGE and DLG containing peptides.
A) and B) The ETGE motif binds to a series of positively charged amino acids in the KEAP1 Kelch domain. The ETGE forms a loop in the binding pose. Two views are shown: from the top A) and the side B). (PDB ID 5WFV). C) and D) The DLG containing peptide shows a pose like the ETGE, but shows fewer contacts, explaining the decreased affinity. The rest of the peptide forms an alpha-helical structure, but this is not known to be physiological or significant due to lack of larger structural data. Two views are shown: from the top C) and the side D). (PDB ID 3WN7).