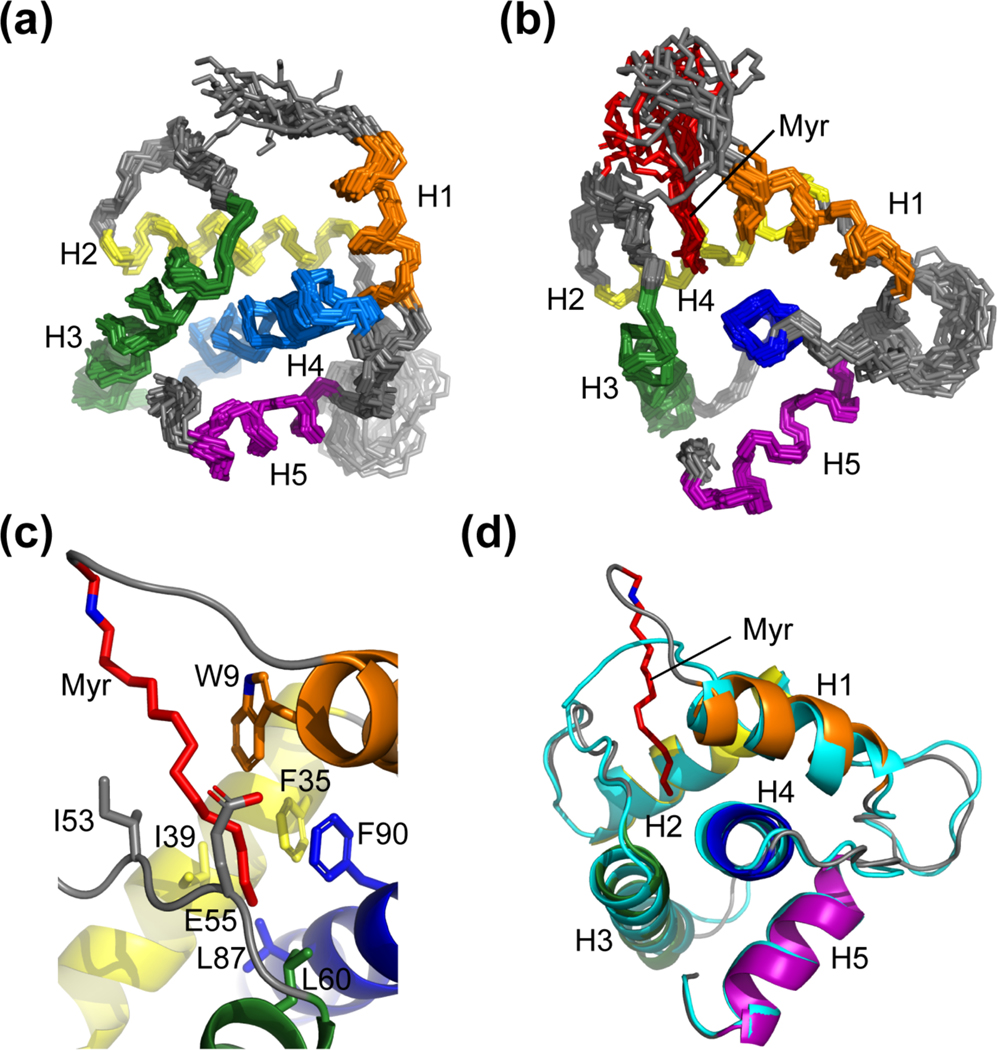
Figure 2.
The NMR structures of the myristoylated and unmyristoylated FIV MA. (a) Overlay of the 20 refined structures calculated for FIV myr(−)MA and (b) FIV MA. Both (a) and (b) are superpositioned based on backbone heavy atoms of the following residues: Arg 7 – Asn 18 (orange), Glu 32 – Thr 46 (yellow), Leu 57 – Phe 74 (green), Ser 77 – Leu 96 (blue), Ala 103 – Met 113 (purple). The orientation of helices and the position of the myristoyl moiety (red) are demonstrated; (c) Residues that compose the hydrophobic pocket to support myristoyl group sequestration; and (d) Ribbon diagram of myr(−)MA (cyan) fitted to the structure of MA, demonstrating that structural differences between the two proteins as observed in the HSQC are localized to the regions that comprise the hydrophobic pocket, where helix I of MA is drawn toward the myristoyl group