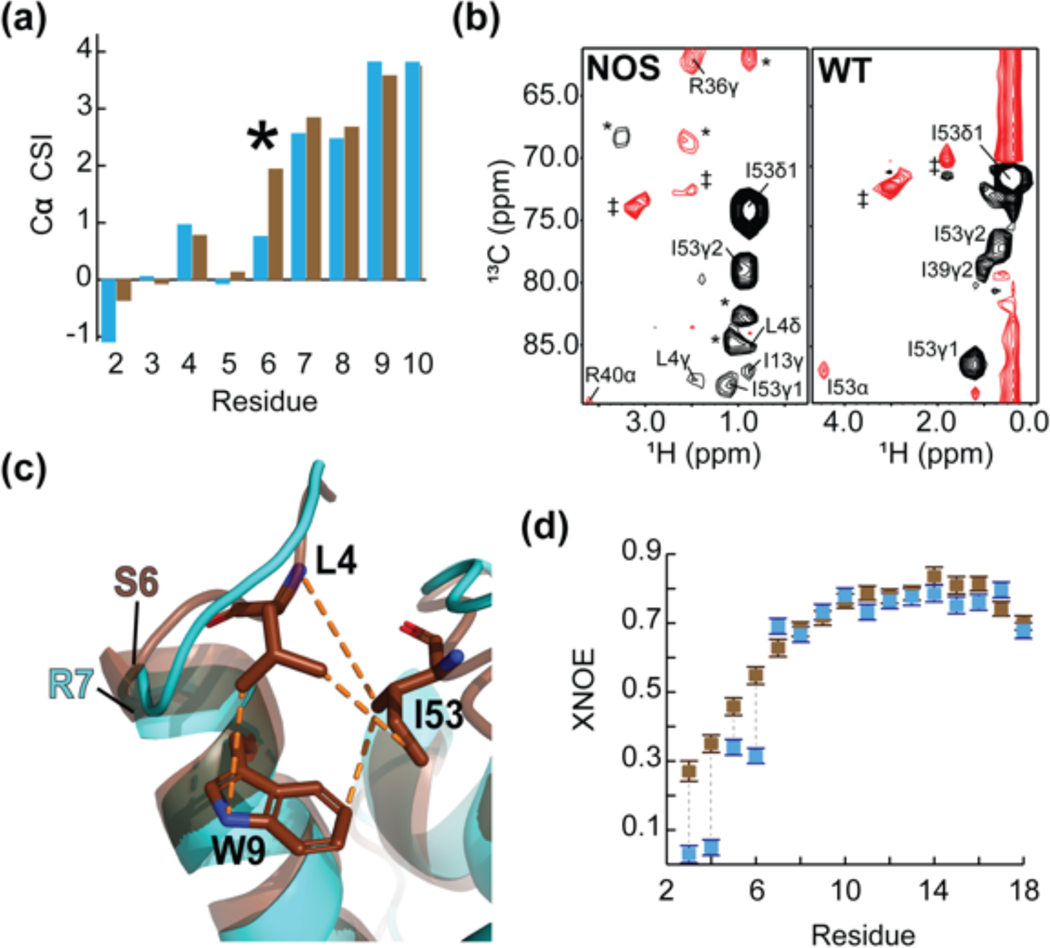
Figure 4.
The consensus feline protein myristoylation signal causes sequestration of FIV MA’s N-terminus. (a) A portion of the Cα CSI indicating that the N-termini of wild-type myr(−)MA and myr(−)MANOS are very similar. Consecutive positive deviations from random coil Cα chemical shift values are suggestive of α-helical secondary structure. In myr(−)MA (cyan), Arg 7 leads helix I as opposed to Ser 6 in myr(−)MANOS (brown); (b) Representative 4D CCNOE spectra of the side chain of Ile 53 in myr(−)MANOS and myr(−)MA demonstrate that the Leu 4 side chain of myr(−)MANOS is sequestered and is close to Ile 53 in comparison to the unstructured Gly 4 in the wildtype myr(−)MA. (* = breakthrough peaks from adjacent planes; ‡ = noise); (c) Ribbon diagrams of myr(−)MA (cyan), and myr(−)MANOS (brown) showing the high degree of structural similarity between the two proteins despite differences in the leading residue of helix I and burial of Leu 4 in myr(−)MANOS; and (d) A portion of the XNOE data of myr(−)MA (cyan) and myr(−)MANOS (brown) showing that the amino-terminus (residues Asn 3 – Gly 6) of myr(−)MA is more mobile than that of myr(−)MANOS whereas first helices of both proteins (residues Arg 7- Asn 18) exhibit similar flexibility.