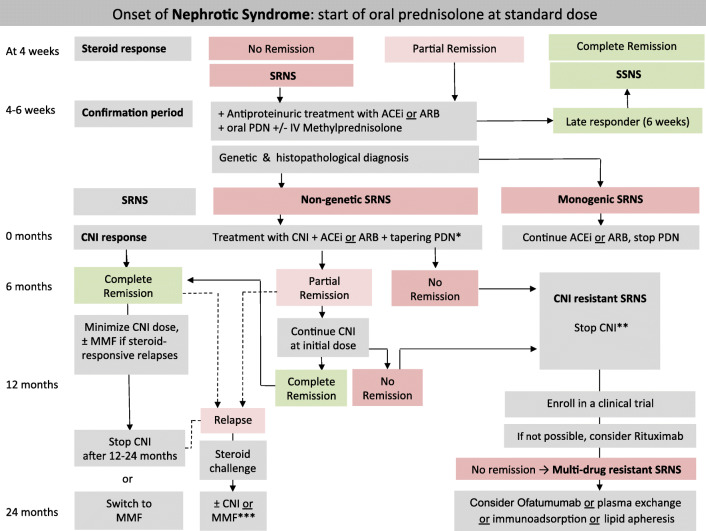
Fig. 2.
Algorithm for the management of children with nephrotic syndrome. Patients are characterized according to response to a 4-week treatment with oral prednisolone (PDN). Patients showing no complete remission enter the confirmation period in which responses to further oral prednisolone (PDN) with or without methylprednisolone (MPDN) pulses in conjunction with either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin-receptor blockers (ARBs) are ascertained and genetic and histopathological evaluation is initiated. Patients with non-genetic SRNS should be candidates for further immunosuppression, whereas those with monogenetic forms are not (further details are given in the text). In the setting of low resource countries where genetic and/or histopathology assessment is not available, immediate immunosuppressive treatment with CNI may be started. If CNI are not available intravenous or oral cyclophosphamide may be started. * = We suggest tapering PDN after CNI initiation as follows: 40 mg/m2 QOD for 4 weeks, 30 mg/m2 QOD for 4 weeks, 20 mg/m2 QOD for 4 weeks, 10 mg/m2 QOD for 8 weeks, and discontinuing thereafter; ** = CNI may be continued in case of partial remission; *** = in cases of no complete response within 4 weeks, frequent relapses or side effects of medications, we recommend following the refractory SRNS protocol; SRNS, steroid-resistant nephrotic syndrome; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; PDN, prednisolone; IV, intravenous; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil