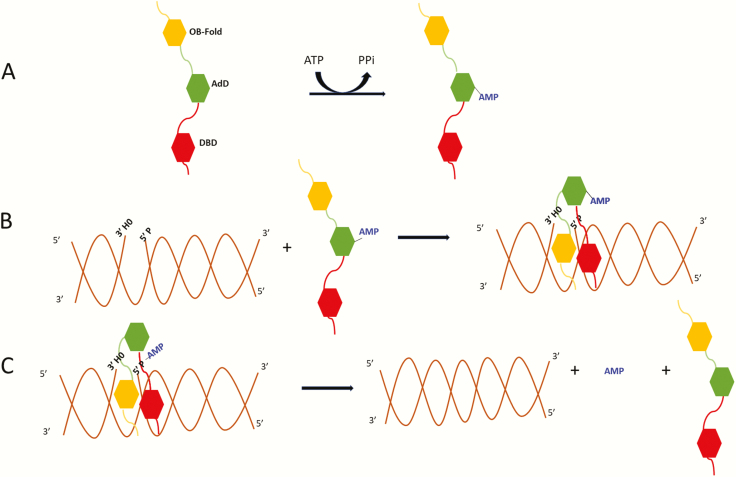
Fig. 1.
Three-step ligation reaction. (A) DNA ligases in an extended conformation interact with ATP to generate a covalent AMP-ligase intermediate with the AMP moiety linked to specific lysine residues in the Adenylation Domain (AdD, green). (B) When the DNA ligase recognises a ligatable nick, the catalytic region composed of the DNA Binding Domain (DBD, red) and Oligonucleotide/Oligosaccharide Binding-fold Domain (OBD, yellow) in addition to the AdD changes conformation, encircling the DNA nick with each of the three domains contacting the DNA. Within this structure, the covalently bound AMP is transferred to the 5′ termini of the nicked DNA. (C) Lastly, using the OH group at the 3′ termini as a nucleophile, non-adenylated DNA ligase catalyses the phosphodiester bond formation releasing the bound AMP.