Figure 4.
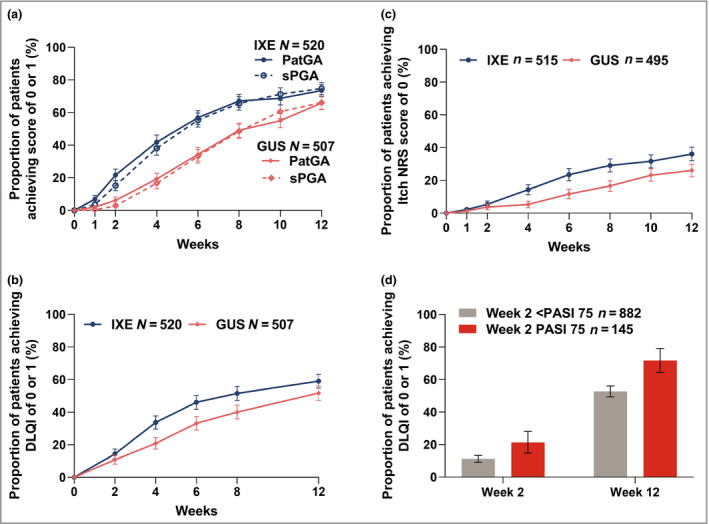
Proportion of patients achieving resolution of patient‐reported outcomes for the ixekizumab (IXE, N = 520) and guselkumab (GUS, N = 507) groups. Data are percentage (with 95% confidence interval). Proportion of patients achieving a score of (a) 0 or 1 for the Patient's Global Assessment (PatGA) and static Physician's Global Assessment (sPGA); (b) 0 or 1 for the Dermatology Life Quality Index (DLQI); and (c) 0 for the itch numeric rating scale (NRS). In (c), only patients with baseline Itch NRS score > 0 were included (IXE n = 515 and GUS n = 495). (d) The proportion of patients achieving DLQI of 0 or 1 based on week 2 75% improvement in Psoriasis Area and Severity Index (PASI 75) is shown after treatment groups were pooled. Nonresponder imputation was used for missing data. The 95% confidence intervals were constructed using the asymptotic method, without continuity correction (i.e. normal approximation to the binomial distribution).
