Figure 1.
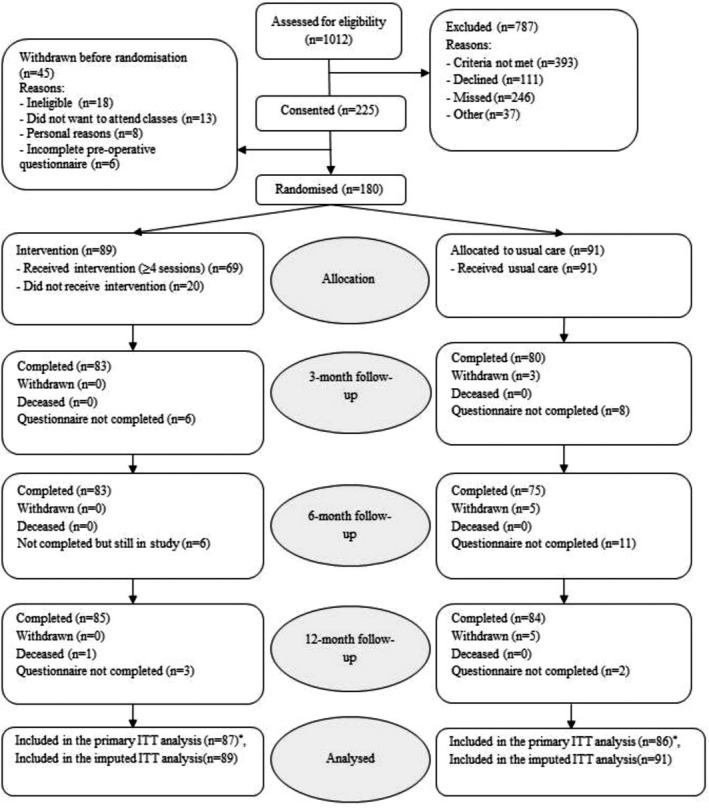
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. * = number of patients who completed at least 1 postoperative Lower Extremity Functional Scale score and therefore were included in the primary mixed regression analysis; ITT = intent‐to‐treat.
