Abstract
Aim
Prenatal exposure to cigarettes leads to alterations in brain development during pregnancy. This has an impact on postnatal psychological and behavioural processes, affecting an infant's neurobehavioural profile with little known about which aspects are affected. The evidence was synthesised to assess the effects of prenatal cigarette smoke exposure on neurobehavioural outcomes within the first year of life.
Methods
Six databases were searched (Web of Science Core Collections, MEDLINE, PsycINFO, CINAHL, EBSCOhost eBook Collection and OpenGrey) in November 2018. Eligible studies (n = 17) had to include a measure of prenatal cigarette exposure and a neurobehavioural assessment ≤1 year of age.
Results
In the first year of life, specific areas of neurobehavioural functioning are related to prenatal cigarette exposure with eight out of 10 areas of neurobehaviour having significant medium (negative affect, attention, excitability, irritability and orientation) or small (muscle tone, regulation and difficult temperament) pooled effect sizes. Only lethargy and stress did not show any significant pooled effects.
Conclusion
Prenatal cigarette exposure affects a significant range of behaviours during the first year of life.
Keywords: meta‐analysis, neurobehaviour, prenatal cigarette exposure, systematic review
Key notes.
Neurobehavioural functioning is affected by prenatal cigarette exposure.
Five areas of neurobehaviour demonstrated significant medium combined effects and three demonstrated significant small combined effects.
Lethargy and stress did not demonstrate significant combined effects.
1. INTRODUCTION
Prenatal exposure to cigarette smoke has lasting postnatal effects including significant increased risk of cognitive impairment and learning difficulties.1, 2, 3 Research suggests two specific toxins in cigarettes are causing these effects, namely carbon monoxide and nicotine. Carbon monoxide crosses the placenta binding to haemoglobin leading to a reduction in blood flow, ultimately impacting brain development and growth.4 Similarly, nicotine readily crosses the syncytium, a thin layer of tissue separating maternal and foetal blood.5 Although the foetal brain is protected from a range of neurotoxins, it is specifically sensitive to nicotine which targets specific neurotransmitters, leading to cell abnormalities and impaired foetal brain development by affecting synaptic activity.5 Since nicotine affects brain development, it has the potential to affect neurobehaviour6 including levels of excitability, negative affect, social orientation and regulation in infants.7 However, there are a number of potential confounding factors that may influence human infant neurobehaviour, leading to difficulties in underpinning the contribution of cigarettes on the neurobehavioural outcome. Therefore, animal models provide an experimental paradigm to define the mechanisms of nicotine on neurobehaviour. For example, where environmental factors are controlled, rats exposed to nicotine show increased motor activity as well as deficits in cognition, including attentional problems.1 From both human and animal research, it appears evident that toxin exposure associated with cigarette smoking leads to alterations in the brain which are reflected in neurobehavioural outcomes.
Neurobehaviour is defined as a bidirectional relationship between biological and behavioural systems, in which behavioural output is moderated by neural feedback.8 It is an interaction between biological and psychosocial factors that influence human behaviour.8 This definition was originally proposed in order to characterise neurobehaviour in late childhood. However, it also applies to infant assessments of neurobehavioural factors such as the availability and fluctuation of sleep and awake states, muscle tone assessed by items such as pulling the infant to a seated position from lying, irritability and neurological reflexes, for example the Babinski and glabellar responses.8, 9 Specific measures assessing infant neurobehavioural development include habituation, muscle tone, attention and stress.10
Measures of infant behavioural development are often not mentioned in information leaflets on the effects of prenatal tobacco exposure which are distributed to parents; rather, leaflets directed at parents emphasise health opposed to psychological risks of smoking.11 Dual emphasis of both the behavioural consequences and health‐related risks associated with smoking is required in order for parents to understand the overall effects of cigarette exposure during pregnancy. Anecdotal experiences of previous healthy uncomplicated pregnancies may lead women to continue smoking during pregnancy.12 However, a thorough understanding of neurobehavioural outcomes within the first year of life and the trajectory of later childhood difficulties is essential information that should be provided to parents before and during their pregnancy. Indeed, research indicates that early neurobehavioural functioning may be predictive of later childhood developmental deficits,13 particularly for infants who have been exposed prenatally to cigarettes.14 There is a growing body of evidence that has assessed the neurobehavioural consequences of prenatal cigarette exposure on infant development during the first year of life.15, 16 Although reviews have been carried out assessing prenatal exposure on developmental outcomes,17, 18 the current review is the first meta‐analysis assessing neurobehavioural outcomes within the first year of life. The emphasis is on the first year of life as insults during the critical period of development may have lasting impact, particularly for behaviour and cognition.19 During prenatal and early infant development, the brain is rapidly changing in regard to structure and function, with toxins, such as metabolites of cigarettes, altering the programming for healthy behavioural development.20 For example, research highlights that scores on a neurobehavioural assessment during infancy had the ability to predict psychomotor development and externalising behaviours at three years of age.21 Moreover, by employing meta‐analytic methods to synthesise the results of the existing studies, we can explore which subcategories of neurobehavioural development are most affected.
2. METHOD AND MATERIALS
The methodological reporting of this review follows the PRISMA guidelines.
2.1. Literature search
In this meta‐analysis, our aim is to identify which subcategories of neurobehaviour are impacted by prenatal cigarette exposure within the first year of life. A literature search of six databases was conducted (Web of Science Core Collections, MEDLINE, PsycINFO, CINAHL, EBSCOhost eBook Collection and OpenGrey) in November 2018. Search terms are listed in Table 1. Although the review focuses on tobacco exposure, nicotine was included as a term to make the search more exhaustive.22
Table 1.
Web of Science Core Collections search strategy
| Search terms | |
|---|---|
| Web of Science Core Collections (k = 1190) 1950‐2018 | |
| Initial search | Maternal smoking pregnancy |
| Prenatal nicotine exposure | |
| Prenatal tobacco exposure | |
| Prenatal cigarette exposure | |
| Prenatal smoke exposure | |
| Foetal nicotine exposure | |
| Foetal tobacco exposure | |
| Foetal cigarette exposure | |
|
Searched within (separately for each phrase) |
Affect (k = 208) Attention (k = 130) Behaviour (k = 127) Cognition* (k = 158) Emotion (k = 62) Excitability (k = 0) Irritability* (k = 4) Lethargy (k = 1) Motor* (k = 46) Muscle tone (k = 7) Neurobehaviour* (k = 30) Neurodevelopment* (k = 53) Orientation (k = 5) Regulation (k = 33) Social (k = 198) Stress (k = 20) Temperament (k = 8) |
| Applicable once duplicates removed: 809 | |
Published articles are restricted from 1950 to 2018, with unpublished research having no time limits. The language was set to English. No methodological limits were applied.
2.2. Study selection
Studies were included if they reported a measure of both prenatal exposure to cigarettes and postnatal neurobehavioural measurements at ≤1‐year post birth. A number of exclusions were in place, including animal studies, reviews (systematic, literature and meta‐analyses), children >1 year of age, studies with no record of maternal prenatal cigarette use, studies focusing on medical, health or birth outcomes and studies using nicotine replacement therapy. The database searches were combined, and duplicate records were removed. The studies were screened by the primary author to assess whether they met the inclusion criteria. Full‐text articles were reviewed for further analysis of study inclusion criteria. The reference lists of these papers were screened for any additional articles. Abstracts and articles were reviewed with the third author.
2.3. Data extraction and assessment of methodological quality
A pre‐defined extraction sheet was used to record study characteristics. Extracted information included (a) main outcome measure, (b) participant characteristics (number of infants, infant age, number prenatally exposed and number not exposed), (c) tobacco measurement, (d) controls and (e) results. Where an effect size (Cohen's d) was not provided, it was calculated from the available data using the Campbell Collaboration effect size calculator (https://campbellcollaboration.org/effect-size-calculato.html). Where possible effect sizes were based on analyses in which potentially confounding variables such as preterm birth, gestational age at birth, maternal demographics, and substance use (eg alcohol),23, 24 had been taken into consideration (Table 2). Risk of bias for individual studies was calculated using the ROBINS‐I tool25 (Table S1).
Table 2.
Studies included within the analysis
| Reference/country | Number of infants | Infant age | Assessment | Subcategory | Effect size (Cohen's d) | Covariates controlled for in the analysis | Overall bias |
|---|---|---|---|---|---|---|---|
|
Barros et al10 Brazil |
388 infants (365 not exposed, 23 exposed) | 24‐72 h old | NICU Network Neurobehavioural Scale | Attention | −1.3 | Anaesthesia at birth, type of delivery, gender, age of newborn at assessment, time since last feed and duration of assessment | Low |
| Excitability | −0.636 | ||||||
| Lethargy | −1.142 | ||||||
| Stress | −0.587 | ||||||
|
Espy et al56 USA |
304 infants (161 not exposed, 143 exposed) | 2 d old | Neonatal Temperament Assessment | Attention | −0.465 | Mothers' IQ estimate. Marital status, maternal age, education, income, alcohol intake, newborn gender, race, SHS exposure, medication use, gravida, parity, weight gain, maternal health, delivery health, BSI summary index, CAARS:S Attention Deficit/Hyperactivity Disorder index and BIA IQ estimate | Low |
| Irritability | −0.192 | ||||||
|
Godding et al57 Belgium |
33 infants (16 not exposed, 17 exposed) | Up to 5 d old | Neurological Scores and Finnegan Withdrawal Scores | Muscle tone | −0.3785 | Term of pregnancy and feeding method | Low |
|
Hernandez‐Martinez et al34 Spain |
265 infants (203 not exposed, 62 exposed) | 48‐72 h old | Neonatal Behavioural Assessment Scale | Negative affect | −0.02 | Socio‐economic status, birthweight and gestational age. Maternal age, socio‐economic status, newborn gender, birthweight, gestational age, Apgar scores, parity, delivery type, trait anxiety | Low |
| Excitability | −0.44 | ||||||
| Orientation | −0.35 | ||||||
| Regulation | −0.351 | ||||||
|
King et al58 USA |
48 infants (24 not exposed, 24 exposed) | 3‐5 mo | Response to bell ring, brain response | Orientation | −0.8471 | Maternal education, gestation at birth, age at assessment, birthweight, ethnicity | Moderate |
|
Law et al32 USA |
56 infants (29 not exposed, 27 exposed) |
Between 36 and 41 wk gestational age | NICU Network Neurobehavioural Scale | Excitability | −0.829 | Parity, 5‐min Apgar scores and birthweight. Maternal age, gravida, education, employment, socio‐economic status, alcohol use, gestational age, Apgar score at 1 min | Low |
| Muscle tone | −0.711 | ||||||
| Stress | −1.510 | ||||||
|
Mansi et al31 Italy |
50 infants (25 not exposed, 25 exposed) | 56‐72 h old | Neonatal Behavioural Assessment Scale | Attention | −1.358 | Gender, gestational age, postnatal age, birthweight, Apgar scores, bilirubin | Low |
| Irritability | −1.949 | ||||||
| Muscle tone | −1.010 | ||||||
| Orientation | −1.115 | ||||||
| Regulation | −0.599 | ||||||
|
Mundy39 UK |
71 infants (47 not exposed, 24 exposed) | 6 mo | Laboratory Temperament Assessment Battery and Infant Behaviour Questionnaire | Difficult temperament | −0.556 | None noted | Moderate |
|
Mundy39 UK |
71 infants (47 not exposed, 24 exposed) | 6 mo | Laboratory Temperament Assessment Battery and Infant Behaviour Questionnaire | Negative affect | −0.409 | None noted | |
| Difficult temperament | −0.399 | ||||||
|
Pickett et al37 UK |
15 943 infants (11 747 not exposed, 4196 exposed) | 9 mo | Carey Infant Temperament Scale | Negative affect | −0.759 | None noted | Moderate |
| Orientation | −0.070 | ||||||
| Regulation | −0.114 | ||||||
| Difficult temperament | −0.134 | ||||||
|
Saxton38 UK |
32 infants (17 not exposed, 15 exposed) | 4‐6 d old | Neonatal Behavioural Assessment Scale | Orientation | −0.8471 | None noted | Moderate |
| Regulation | −0.782 | ||||||
|
Schuetze et al29 USA |
115 infants (46 not exposed, 69 exposed) | 2‐4 wk old and again at 7 mo old | Infant Behaviour Questionnaire | Negative affect | −0.806 | Mothers' age, education, socio‐economic status, parity, number of prenatal visits, substance use, infant birthweight, head circumference and birth length | Low |
|
Shisler et al30 USA |
258 infants (77 not exposed, 181 exposed) | 2 and 9 mo old | Focused attention assessment and behavioural reactivity | Attention | −0.238 | Mothers' age, education, prenatal alcohol and marijuana, partner status, birthweight, gestational age, gender, head circumference at birth | Low |
|
Stroud et al35 USA |
56 infants (28 not exposed, 28 exposed) | 17 d old | NICU Network Neurobehavioural Scale | Excitability | −0.665 | Maternal SHS exposure, infant SHS exposure, feeding, maternal depression, socio‐economic status, maternal age and depression | Low |
| Regulation | −0.565 | ||||||
|
Stroud et al48 USA |
962 infants (366 not exposed, 596 exposed) | <3 d old | Graham‐Rosenblith Behavioural Examination | Irritability | −0.125 | Maternal age, race, socio‐economic status, birthweight and infant age at assessment. Gravida, parity, Apgar score at 1 min and Apgar score at 5 min | Low |
| Muscle tone | −0.308 | ||||||
|
Wiebe et al59 USA |
218 infants (91 not exposed, 127 exposed) | 6 mo old | A battery of assessments including attention, regulation and inhibition | Orientation | −0.236 | Propensity scores—alcohol in first month of pregnancy, maternal age, education, IQ, hyperactivity. Parental stress and infant exposure | Moderate |
|
Yolton et al22 USA |
251 infants (218 not exposed, 33 exposed) | 5 wk old | NICU Network Neurobehavioural Scale | Attention | −0.134 | Birthweight, age at assessment and infant gender. Maternal age, income, employment, education, marital status, parity, marijuana and alcohol use, maternal blood lead in pregnancy and weight change since birth and maternal depression | Low |
| Lethargy | −0.147 | ||||||
| Regulation | −0.067 | ||||||
| Stress | −0.002 |
2.4. Data analysis
Studies that were eligible for the review were grouped according to 10 different subcategories of outcome measures: negative affect, attention, excitability, irritability, lethargy, muscle tone, orientation, regulation, stress and difficult temperament. To be included in the meta‐analysis, the assessment measures had to be similar across the subcategory. For subcategories of neurobehaviour to be included within the analysis, two or more studies were required.26 The fail‐safe N method was used to identify any publication bias by providing an estimate of the number of missing studies that would need to be published with an effect size of d = 0 for the pooled effect size to not be significant.27
3. RESULTS
3.1. Selection of studies
The search resulted in 2208 studies. After removal of duplicates, 854 studies were reviewed in terms of title and abstract, resulting in 49 eligible studies which were subjected to a full‐text review. These articles were reviewed in‐depth, checking for a measure of prenatal smoke exposure and a postnatal neurobehavioural measure, and 27 articles were removed leaving 22 articles that based on our selection criteria could be included in the review (see Figure 1). Five of these articles reported insufficient data leaving 17 articles included in the meta‐analysis. Authors of the five studies reporting insufficient results were contacted, where possible, to obtain further details. However, this was unsuccessful. See Figure 1 for flow diagram of study selection and Table 2 for details of the studies included in the analysis.
Figure 1.
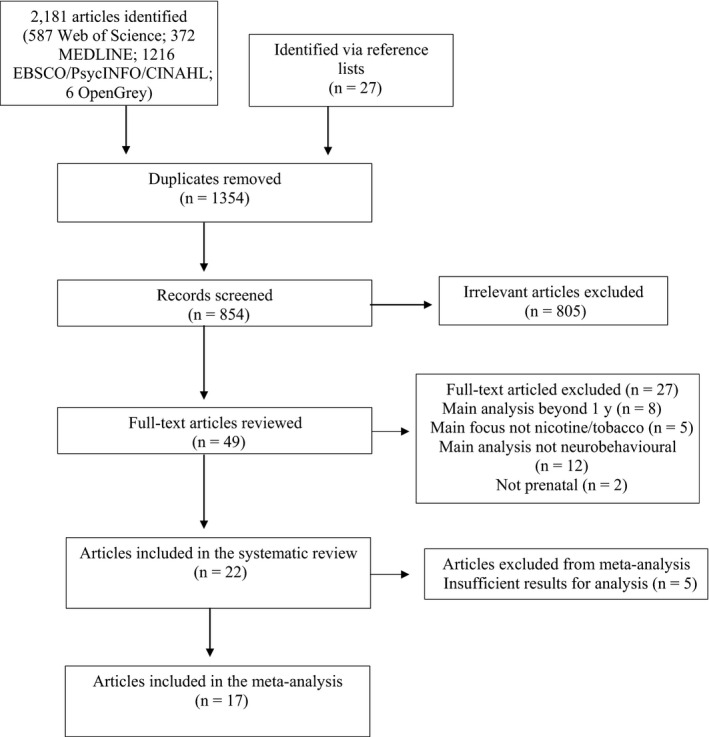
PRISMA flow diagram of studies
3.2. Study characteristics
The 17 studies included in the meta‐analysis analysed 19 162 infants. There were 5672 infants exposed to cigarettes prenatally and 13 490 who had no prenatal cigarette exposure. Studies came from six different countries: USA (n = 9), UK (n = 4), Spain (n = 1), Italy (n = 1), Brazil (n = 1) and Belgium (n = 1). To assess level of maternal or infant smoke exposure, studies used either a questionnaire method (n = 7), biological measures such as cotinine levels via saliva (n = 2) or a combination of the two methods (n = 8). Nine different assessment scales were used to measure a range of neurobehaviours. Details of the assessments are in Table 3.
Table 3.
Assessment measures
| Assessment measure | Number of studies using assessment | Details |
|---|---|---|
| NICU Network Neurobehavioural Scale (NNNS) | 4 | This assessment was designed to capture the vulnerabilities of high‐risk infants exposed to toxic substances and for newborns between 30 and 46 wk gestational age. Raw data were used to create summary scores based on 13 dimensions including attention, arousal, excitability, hypertonicity, hypotonicity, lethargy, regulation, handling, stress and reflexes22 |
| Neonatal Behaviour Assessment Scale (NBAS) | 3 | Assesses early regulatory behaviour.56 State changes are provoked and the infants' habituation, self‐consoling abilities and reflexes. It includes 28 behavioural items and 18 reflexes. Items given a score include motor abilities, habituation, orientation, reflexes and regulation31 |
| Carey Infant Temperament Scale | 1 | The scale assesses three areas of temperament: positive mood, receptivity to novelty and regularity37 |
| Infant Behaviour Questionnaire‐Revised | 2 | This is a parental report questionnaire for infants between 3 and 12 mo of age. There are four main subcategories of this scale including extroversion, negative affect, orientation and regulation28 |
| Graham‐Rosenblith Behavioural Examination | 1 | This is a standardised assessment which involves observation and manipulation of the infant to assess reflexes, muscle tone and responses to stimulation. Additionally, measures of irritability and signs of neurological damage are assessed35 |
| Laboratory Assessment Battery (Lab‐TAB) | 2 | Designed to assess early infant temperament39 |
| Finnegan Withdrawal Scale | 1 | Evaluation of the central nervous system function and respiratory functions57 |
| Neurological Scores | 1 | Assesses a range of abilities including muscle tone, reflexes, for example sucking, stepping reactions and alertness, for example eye opening57 |
| Neonatal Temperament Assessment (NTA) | 1 | The assessment assesses early regulatory behaviours56 |
3.3. Neurobehavioural subcategory analysis
See Figure 2 for forest plot of results and Table 4 for subcategory analysis.
Figure 2.
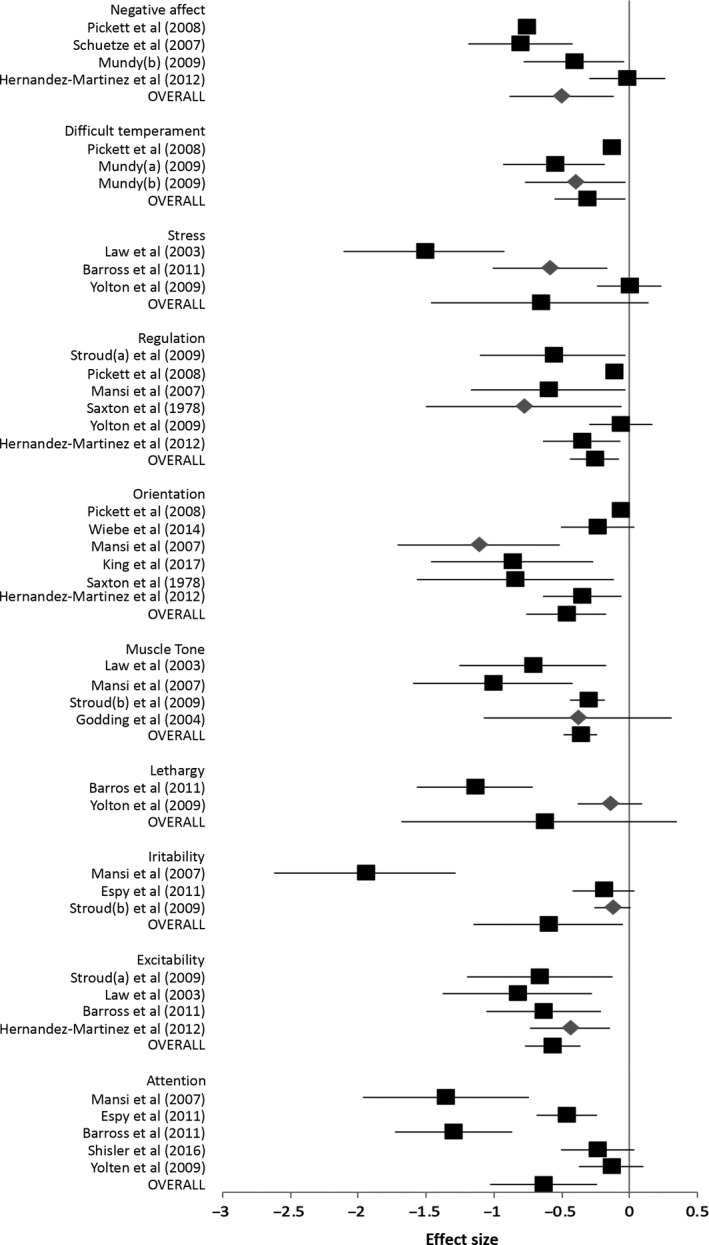
Forest plot of analysis
Table 4.
Subcategory analysis
| Subcategory | Number of studies | Assessment measures | Cohen's d | 95% CI | Z | P value (Z) | Q | P value (Q) |
|---|---|---|---|---|---|---|---|---|
| Negative affect* | 4 | NBAS, Lab‐TAB, Carey Infant Temperament Scale, Infant Behaviour Questionnaire‐Revised | −0.5027 | −0.8863, −0.1191 | −2.5685 | .0102 | 28.2227 | <.001 |
| Attention* | 5 | NBAS, NICU Network Neurobehavioural Scale, NTA | −0.6352 | 1.0318, −0.2386 | −3.1292 | .001 | 32.4514 | <.001 |
| Excitability* | 4 | NICU Network Neurobehavioural Scale, NBAS | −0.5697 | −0.7726, −0.3678 | −5.5296 | <.001 | 1.8737 | .599 |
| Irritability* | 3 | NICU Network Neurobehavioural Scale, Graham‐Rosenblith Behavioural Examination, NTA | −0.6003 | −1.1486, −0.0519 | −2.1456 | .0319 | 27.185 | <.001 |
| Lethargy | 2 | NICU Network Neurobehavioural Scale | −0.6280 | −1.680, 0.3469 | −1.2625 | .2068 | 15.8478 | .001 |
| Muscle tone* | 4 | NICU Network Neurobehavioural Scale, Graham‐Rosenblith Behavioural Examination, NBAS, Neurological Scores | −0.3619 | −0.4842, −0.2395 | −5.7964 | <.001 | 6.9088 | .0749 |
| Orientation* | 6 | NBAS, Carey Infant Temperament Scale | −0.4645 | ‐0.7577, 0.1713 | −3.1047 | .001 | 26.9692 | .009 |
| Regulation* | 6 | NICU Network Neurobehavioural Scale, NBAS, Carey Infant Temperament Scale | −0.2619 | −0.4411, −0.0827 | −2.864 | .004 | 11.2507 | .0465 |
| Stress | 3 | NICU Network Neurobehavioural Scale | −0.6613 | −1.4598, 0.1373 | −1.6231 | .1046 | 23.7939 | <.001 |
| Difficult temperament* | 3 | Lab‐TAB, Carey Infant Temperament Scale | −0.3144 | −0.5966, −0.0322 | −2.1834 | .0290 | 6.567 | .0369 |
If the Q statistic was significant (P < .05), the random effects size model was used to compute the pooled effect size. If the Q statistic was not significant (P > .05), the fixed effects size model was used to compute the pooled effect size.
Significant P < .05.
3.4. Negative affect
Negative affect is determined by establishing level of sadness, fear, soothability and activity level28 and is linked to the infant's ability to regulate their emotional state. Four studies were included in the analysis of negative affect. A total of 16 394 infants (12 043 not exposed and 4351 exposed) between 48 hours and 9 months old were assessed on one of four measures: NBAS, Lab‐TAB, Carey Infant Temperament Scale and Infant Behaviour Questionnaire‐Revised. Individual study effect sizes ranged between −0.80629 and −0.02.7 Due to heterogeneity within the sample (Q = 28.222, P < .001, I 2 = 89.37%), the random effects size model is reported. The combined effect size for negative affect is significant (d = −0.502; 95% CI = −0.886 to −0.1191; z = −2.568, P = .010; fail‐safe N = 809). Infants prenatally exposed to smoking showed heightened negative affect.
3.5. Attention
Infant attentional abilities are assessed by the degree of energy the infant displays when engaging with the assessment and the level of facilitation required from the examiner to gain the infant's attention.30 Five studies were included in the assessment of the attention subcategory, assessing 1251 infants (846 not exposed to nicotine and 405 exposed to nicotine), between 24 hours and 9 months old. Three different assessment scales were used: NBAS, NICU Network Neurobehavioural Scale and NTA. Individual study effect sizes ranged between −1.35831 and −0.134,22 and there is evidence of heterogeneity within the sample (Q = 32.451, P < .001, I 2 = 87.67%). Therefore, the random effects size model is reported. The combined effect size for attention is significant (d = −0.635; 95% CI = −1.031 to −0.238; z = −3.129, P = .001; fail‐safe N = 98). Those exposed to cigarettes showed significantly poorer levels of attention.
3.6. Excitability
Excitability measures peak excitement and rapidity of build‐up, which is a reflection of how much stimulation the baby can handle before entering the crying state, indicating higher levels of arousal.32, 33 A total of 765 infants (625 not exposed and 140 exposed) between 24 hours and 17 days old were included in the four studies analysed for excitability using two different assessment scales (NICU Network Neurobehavioural Scale and the NBAS). Individual study effect sizes ranged between −0.82932 and −0.44.34 The data are homogeneous (Q = 1.873, P = .599, I 2 = 60.13%), and therefore, the fixed effects size model is reported. The combined effect size for excitability is significant (d = −0.5697; 95% CI = −0.772 to −0.367; z = −5.529, P < .001; fail‐safe N = 44). Infants prenatally exposed to cigarettes demonstrated significantly higher levels of excitability.
3.7. Irritability
Irritability is assessed by examining the amount of fussing and crying throughout neurobehavioural assessments, again a reflection of their emotional capabilities. Three studies were included in the analysis for irritability with 1316 (552 not exposed and 764 exposed) infants between 56 hours and 3 days old. The NICU Network Neurobehavioural Scale, Graham‐Rosenblith Behavioural Examination and NTA were used. Individual study effect sizes ranged between −1.94931 and −0.125.35 The random effects size model was used because of heterogeneity within the data (Q = 27.185, P < .001, I 2 = 92.64%). The combined effect size for irritability was significant (d = −0.600; 95% CI = −1.148 to −0.0519; z = −2.145, P = .031; fail‐safe N = 29). Infants prenatally exposed to cigarettes were significantly more irritable.
3.8. Lethargy
Lethargy measures indicate the energy resources of the infant and are identified by items on the neurobehavioural assessments such as general muscle tone and reaction to the defensive movement by establishing level of movement.33 Two studies were included in the analysis for lethargy with 639 infants (583 not exposed and 56 exposed) ranging between 24 hours and 5 weeks in age, tested with the NICU Network Neurobehavioural Scale. Individual study effect sizes ranged from −1.14210 to −0.147.22 The data are heterogeneous (Q = 15.847, P < .00, I 2 = 93.68%); therefore, the random effects size model is reported. The combined effect size for lethargy is not significant (d = −0.628; 95% CI = −1.680 to 0.346, z = −1.262, P = .206). Prenatal exposure to smoking is not significantly related to the lethargy levels of infants tested.
3.9. Muscle tone
Muscle tone is identified by assessing how smooth or jerky the infant's movements are and amount of 90° arm movements the infant displays. Additionally, measures such as pulling the infant to sit are used as an indication of muscle tone.33 Muscle‐tone weakness is identified in the infant when the majority of movements are jerky, restricted and when there is significant head lag when the infant is pulled to a seated position.36 Four studies were included in the analysis for muscle tone with a total of 1101 infants (436 not exposed and 665 exposed), between 56 hours and 5 days old assessed with one of four measures (NICU Network Neurobehavioural Scale, Graham‐Rosenblith Behavioural Examination, NBAS and Neurological Scores). Individual studies had an effect size ranging between −1.01031 and −0.308.35 The data were homogeneous (Q = 6.908, P = .074, I 2 = 56.57%); therefore, the fixed effects size model is reported. The combined effect size is significant (d = −0.361; 95% CI = −0.484 to −0.239; z = −5.796, P < .001; fail‐safe N = 28). Infants prenatally exposed to smoking had significantly more muscle‐tone weakness.
3.10. Orientation
Orientation items assess the infant's ability to follow and engage with animate and inanimate objects such as following a face or rattle for example.33 A total of 16 556 infants (12 107 not exposed and 4449 exposed) between 48 hours and 9 months old, based on six studies, were included in the subcategory analysis for orientation. The assessments used were the NBAS and Carey Infant Temperament Scale. The range of effect sizes across individual studies was −1.11531 and −0.070.37 Due to heterogeneity (Q = 26.969, P = .001, I 2 = 81.46%) of the sample, the random effects size model is reported. The combined effect size for orientation is significant (d = −0.464; 95% CI = −0.757 to −0.171; z = −3.104, P < .001; fail‐safe N = 98). Infants prenatally exposed to smoking demonstrated significantly worse levels of orientation.
3.11. Regulation
Regulation is assessed by the infant's abilities to self‐sooth, for example whether they need support in settling down following a period of crying,33 emphasising their emotional self‐soothing abilities. A total of 16 597 infants (12 238 not exposed and 4359 exposed), between 48 hours and 9 months old, were analysed in the subcategory for regulation, based on six studies using three different assessment measures (NICU Network Neurobehavioural Scale, NBAS and Carey Infant Temperament Scale). Individual study effect sizes ranged between −0.78238 and −0.067.22 This was a heterogeneous sample (Q = 11.250, P = .046, I 2 = 55.55%), and therefore, the random effects size model is reported. The combined effect size for orientation abilities was significant (d = −0.261 (95% CI = −0.4411 to −0.082; z = −2.864, P = .004; fail‐safe N = 82). Infants prenatally exposed to smoking showed significantly more problems in their ability to regulate their behaviour.
3.12. Stress
Infant stress is a reflection of the autonomic nervous system and as such is determined by whether colour changes in the face or body occur, number of startles and whether tremors can be seen throughout the assessment.33 A total of 695 infants (612 not exposed and 83 exposed), between 24 hours and 5 weeks old, were tested using a single assessment measure, the NICU Network Neurobehavioural Scale, across three studies. Individual study effect sizes varied between −1.51032 and −0.002.22 Due to heterogeneity in the sample (Q = 23.793, P < .001, I 2 = 91.59%), the random effects size model was used. The combined effect size for stress was not significant (d = −0.661; 95% CI = −1.459 to 0.137; z = −1.623, P = .104). Infants prenatally exposed to smoking did not show significantly higher stress compared with non‐exposed infants.
3.13. Difficult temperament
Difficult temperament of the infant, that is fussiness, irritability and negative affect throughout the assessment, is used to determine the infant's temperament.29 A total of 192 infants (116 not exposed and 73 exposed) between 56 and 6 months old were assessed in three studies using the Lab‐TAB and the Carey Infant Temperament Scale for temperament. Individual studies reported effect sizes between −0.55639 and −0.134.37 Because of the heterogeneity within the sample (Q = 6.596, P = .036, I 2 = 69.68%), the random effects size model was used. The combined effect size for temperament was significant (d = −0.314; 95% CI = −0.596 to −0.032; z = −2.183, P = .029; fail‐safe N = 14). Infants prenatally exposed to cigarette smoke demonstrated higher levels of difficult temperament in comparison with infants not prenatally exposed to smoke.
4. DISCUSSION
The aim of this systematic review and meta‐analysis was to establish which areas of neurobehaviour are most strongly related to prenatal cigarette exposure in infants up to one year of age. Overall, the results support the claim that prenatal exposure to smoking is associated with a range of neurobehavioural consequences in infants within the first year of life. Eight of the 10 subcategories that were analysed in the meta‐analysis indicate that prenatal smoking is significantly associated with poorer neurobehavioural functioning in infancy. Measures of negative affect, attention, excitability, irritability and orientation demonstrated medium significant effects, with regulation, difficult temperament and muscle‐tone weakness, indicating smaller significant effects. Stress and lethargy tests, however, did not result in any significant pooled effects.
We argue that the neurobehavioural deficits evident in infants of mothers who smoke cigarettes reflect early behavioural dysregulation associated with prenatal exposure to cigarette smoking. The metabolites of cigarette smoke, carbon monoxide and nicotine, interfere with the normal placental functioning acting as a vasoconstrictor, with uterine blood flow being restricted to roughly 38%.4, 40, 41 Carbon monoxide is likely to lead to foetal hypoxia depriving the developing brain of oxygen and nutrients required for typical brain development. Such effects can be seen in prenatally exposed newborns whose cerebral oxygen saturation level is lower in comparison with infants not exposed.42 This interpretation is supported by studies using animal models.43, 44 Similarly, studies highlight the widespread effects of nicotine affecting a range of neurotransmitters, brain regions and systems which disrupt brain development. Specifically, the neurotransmitter nicotine acetylcholine plays a role in supporting the development of infant regulatory behaviours, such as temperament.35, 43 Differences in neurobehaviour of infants prenatally exposed to cigarettes are based on changes in brain functioning as a result of carbon monoxide and nicotine exposure.4
Research indicates that mother‐infant relationships are under more stress, that is less responsiveness and emotional interactions, if the infant displays neurobehavioural deficits in areas such as affect, with infants demonstrating reduced eye contact and/or reduced smiling during parent‐infant interaction.45 This type of unresponsiveness by the infant leads to a negative feedback loop during mother‐infant interactions. As this review indicates, maternal smoking during pregnancy is related to deficits in infant neurobehavioural functioning; for example, infants prenatally exposed to cigarettes are likely to be more irritable compared to non‐exposed infants. A more irritable child will affect quality of parenting behaviours which have negative effects on the infant including less stimulation, less responsiveness and less physical contact.46 Because of these negative parenting engagements, the infant's neurobehavioural development is further dysregulated due to reduced interactions.31 As a result, an infant who lacks stimulation and physical contact is more likely to show delays in their motor development.47 This delay in turn will be an additional strain on the already stressed mother‐infant relationship. Long‐term attentional and behavioural problems can be reflective of these early deficits in neurobehavioural functioning of an infant.48
4.1. Limitations
The relationship between neurobehavioural developmental factors and prenatal cigarette smoke exposure is complex, often associated with a number of covariates such as preterm birth, gestational age at birth, maternal demographics and substance use (eg alcohol).23, 24 As shown in Table 2, these types of variables were controlled for in the effect size analysis in the majority of studies. Nevertheless, other covariates such as maternal psychological factors were not considered in many of the studies reviewed, despite the known effects on infant neurobehaviour. For example, maternal antenatal stress and anxiety are positively related to infant outcomes including behavioural and cognitive development such as regulation difficulties, irritability and poorer attention.49 Given that these factors were not controlled for in all the studies analysing the effect of cigarette exposure, it was difficult to determine in our current review the extent to which these factors may have influenced the test results.
Due to such confounding variables, it is possible that studies claiming to find a relationship between prenatal smoke exposure and subsequent infant neurobehaviour are measuring an indirect relationship rather than a true causal effect.50, 51 As a consequence of the epidemiological nature of this research, not all potential confounds can be controlled for and it is difficult to carry out a true experimental design as cigarette exposure cannot be randomly assigned, thus highlighting a methodological limitation.52 However, by synthesising the available evidence across multiple populations and study designs, this meta‐analysis strengthens the case for a true causal effect between cigarette exposure and infant neurobehaviour.50, 51
It is notable however that by studying infants up to one year of age (the range of ages of infants studied is shown in Table 2), we cannot rule out the possibility that in the older infants the effects of their mothers' smoking on neurobehavioural outcomes were due to postnatal rather than prenatal exposure.9 Furthermore, the amount of cigarette exposure and at what time point exposure occurred (including postnatal exposure) differed between studies. In the early stage of development, there is naturally a lot of variation and disorganisation in the neurobehavioural profile of infants since the brain is not fully developed at birth,53 and environmental factors influence brain development.54 Therefore, we have to consider whether the differences seen in infant neurobehavioural development are short‐term or long‐term factors and whether the negative consequences can be reduced or potentially eliminated through neurobehavioural interventions.
5. CONCLUSIONS
The results from the meta‐analysis indicate that exposure to prenatal cigarette smoking is associated with negative neurobehavioural outcomes in infants up to one year of age. Research indicates that not all women believe that smoking has negative behavioural consequences for their infant.55 Thus, examining neurobehavioural differences in smoke‐exposed and non‐exposed foetuses and infants is essential in order to convince pregnant women to abstain from cigarette consumption during their pregnancy and after birth. For example, smoking during pregnancy may result in irritable infants which cry more than infants with a calm temperament.37 The current review and analysis provides further support of the negative effects prenatal smoke exposure has on infant neurobehaviour within the first year of life.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Professor Vivette Glover for her helpful comments on the paper.
Biographies
Suzanne Froggatt

Judith Covey

Nadja Reissland

Froggatt S, Covey J, Reissland N. Infant neurobehavioural consequences of prenatal cigarette exposure: A systematic review and meta‐analysis. Acta Paediatr. 2020;109:1112–1124. 10.1111/apa.15132
Funding information
This work was funded by the Economic and Social Research Council. The funding source had no involvement with the design, analysis, interpretation or writing of this report.
REFERENCES
- 1. Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40(6):630‐641. [DOI] [PubMed] [Google Scholar]
- 2. Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931‐945. [PubMed] [Google Scholar]
- 3. Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92(6):966‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015;104(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 5. Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001;24(4):277‐322. [DOI] [PubMed] [Google Scholar]
- 6. Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernández‐Martínez C, Val VA, Subias JE, Sans JC. A longitudinal study on the effects of maternal smoking and secondhand smoke exposure during pregnancy on neonatal neurobehaviour. Early Human Dev. 2012;88(6):403‐408. [DOI] [PubMed] [Google Scholar]
- 8. Lester BM, Tronic EZ. The neonatal intensive care unit network neurobehavioral scale procedures. Pediatrics. 2004;113:641‐667. [PubMed] [Google Scholar]
- 9. Xu Y, Yolton K, Khoury J. Earliest appropriate time for administering neurobehavioral assessment in newborn infants. Pediatrics. 2011;127(1):69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barros MCM, Mitsuhiro SS, Chalem E, Laranjeira RR, Guinsburg R. Prenatal tobacco exposure is related to neurobehavioral modifications in infants of adolescent mothers. Clinics. 2011;66(9):1597‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. NHS . Stop Smoking in Pregnancy; 2016. https://www.nhs.uk/conditions/pregnancy-and-baby/smoking-pregnant/. Updated October 20, 2016. [Google Scholar]
- 12. Haslam C, Draper ES. A qualitative study of smoking during pregnancy. Psychol Health Med. 2001;6(1):95‐99. [Google Scholar]
- 13. Liu J, Bann C, Lester B, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125(1):E90‐E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24‐41. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez‐Martinez C, Moreso NV, Serra BR, Val VA, Macias JE, Sans JC. Effects of prenatal nicotine exposure on infant language development: a cohort follow up study. Matern Child Health J. 2017;21(4):734‐744. [DOI] [PubMed] [Google Scholar]
- 16. Stroud LR, Papandonatos GD, Rodriguez D, et al. Maternal smoking during pregnancy and infant stress response: test of a prenatal programming hypothesis. Psychoneuroendocrinology. 2014;48:29‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 2009;22(2):121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olds DL, Eckenrode J, Henderson CR, et al. Long‐term effects of home visitation on maternal life course and child abuse and neglect ‐ fifteen‐year follow‐up of a randomized trial. JAMA J Am Med Assoc. 1997;278(8):637‐643. [PubMed] [Google Scholar]
- 19. Stettler N. Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. Int J Obes. 2007;31(7):1035. [DOI] [PubMed] [Google Scholar]
- 20. Anderson AL, Thomason ME. Functional plasticity before the cradle: a review of the neural functional imaging in the human fetus. Neurosci Biobehav Rev. 2013;37(9):2220‐2232. [DOI] [PubMed] [Google Scholar]
- 21. Sucharew H, Khoury JC, Xu Y, Succop P, Yolton K. NICU Network Neurobehavioral Scale profiles predict developmental outcomes in a low‐risk sample. Paediatr Perinatal Epidemiol. 2012;26(4):344‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yolton K, Khoury J, Xu Y, et al. Low‐level prenatal exposure to nicotine and infant neurobehavior. Neurotoxicol Teratol. 2009;31(6):356‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Field T, Diego M, Dieter J, et al. Prenatal depression effects on the fetus and the newborn. Infant Behavior Dev. 2004;27(2):216‐229. [DOI] [PubMed] [Google Scholar]
- 24. Lipper E, Lee KS, Gartner LM, Grellong B. Determinants of neurobehavioral outcome in low‐birth‐weight infants. Pediatrics. 1981;4(67):502‐505. [PubMed] [Google Scholar]
- 25. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta‐analysis. J Educ Behav Stat. 2010;35(2):215‐247. [Google Scholar]
- 27. Rosenthal R. Combining results of independent studies. Psychol Bull. 1978;85(1):185. [Google Scholar]
- 28. Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behaviour questionnaire. Infant Behavior Dev. 2003;26(1):64‐86. [Google Scholar]
- 29. Schuetze P, Eiden RD. The association between prenatal exposure to cigarettes and infant and maternal negative affect. Infant Behavior Dev. 2007;30(3):387‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shisler S, Eiden RD, Molnar DS, et al. Effects of fetal tobacco exposure on focused attention in infancy. Infant Behavior Dev. 2016;45:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mansi G, Raimondi F, Pichini S, et al. Neonatal urinary cotinine correlates with behavioral alterations in newborns prenatally exposed to tobacco smoke. Pediatr Res. 2007;61(2):257‐261. [DOI] [PubMed] [Google Scholar]
- 32. Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6):1318‐1323. [DOI] [PubMed] [Google Scholar]
- 33. Tronick E, Lester BM. Grandchild of the NBAS: the NICU network neurobehavioral scale (NNNS): a review of the research using the NNNS. J Child Adolescent Psychiatric Nurs. 2013;26(3):193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hernandez‐Martinez C, Arija Val V, Escribano Subias J, Canals SJ. A longitudinal study on the effects of maternal smoking and secondhand smoke exposure during pregnancy on neonatal neurobehavior. Early Human Dev. 2012;88(6):403‐408. [DOI] [PubMed] [Google Scholar]
- 35. Stroud LR, Paster RL, Goodwin MS, et al. Maternal smoking during pregnancy and neonatal behavior: a large‐scale community study. Pediatrics. 2009;123(5):E842‐E848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brazelton TB, Nugent JK, Lester BM. Neonatal behavioral assessment scale. [Google Scholar]
- 37. Pickett KE, Wood C, Adamson J, D'Souza L. Meaningful differences in maternal smoking behaviour during pregnancy: implications for infant behavioural vulnerability (vol 62, pg 318, 2008). J Epidemiol Commun Health. 2008;62(5):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saxton DW. The behaviour of infants whose mothers smoke in pregnancy. Early Human Dev. 1978;2(4):363‐369. [DOI] [PubMed] [Google Scholar]
- 39. Mundy LK. Infant attention, motor activity and cardiac activity and the effects of prenatal smoke exposure; 2009. [Google Scholar]
- 40. Bush PG, Mayhew TM, Abramovich DR, Aggett PJ, Burke MD, Page KR. Maternal cigarette smoking and oxygen diffusion across the placenta. Placenta. 2000;21(8):824‐833. [DOI] [PubMed] [Google Scholar]
- 41. Suzuki K, Minei LJ, Johnson EE. Effect of nicotine upon uterine blood flow in the pregnant rhesus monkey. Am J Obstet Gynecol. 1980;136(8):1009‐1013. [DOI] [PubMed] [Google Scholar]
- 42. Verhagen EA, ter Horst HJ, Kooi EMW, Keating P, van den Berg PP, Bos AF. Prenatal tobacco exposure influences cerebral oxygenation in preterm infants. Early Human Dev. 2011;87(6):401‐406. [DOI] [PubMed] [Google Scholar]
- 43. Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30(1):1‐19. [DOI] [PubMed] [Google Scholar]
- 44. Cohen G, Roux J‐C, Grailhe R, Malcolm G, Changeux J‐P, Lagercrantz H. Perinatal exposure to nicotine causes deficits associated with a loss of nicotinic receptor function. Proc Natl Acad Sci USA. 2005;102(10):3817‐3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Papoušek M, von Hofacker N. Persistent crying in early infancy: a non‐trivial condition of risk for the developing mother‐infant relationship. Child Care Health Dev. 1998;24(5):395–424. [DOI] [PubMed] [Google Scholar]
- 46. van den Bloom DC, Hoeksma JB. The effect of infant irritability on mother‐infant interaction: a growth‐curve analysis. Dev Psychol. 1994;30(4):581. [Google Scholar]
- 47. Gutman LM, Feinstein L. Parenting behaviours and children's development from infancy to early childhood: changes, continuities and contributions. Early Child Dev Care. 2010;180(4):535‐556. [Google Scholar]
- 48. Stroud LR, Paster RL, Papandonatos GD, et al. Maternal smoking during pregnancy and newborn neurobehavior: effects at 10 to 27 days. J Pediatr. 2009;154(1):10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29(2):237‐258. [DOI] [PubMed] [Google Scholar]
- 50. Grimes DA, Schulz KF. Bias and causal associations in observational research. The Lancet. 2002;359(9302):248‐252. [DOI] [PubMed] [Google Scholar]
- 51. Brion M‐J, Victora C, Matijasevich A, et al. Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics. 2010;126(1):e57‐e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D'Onofrio BM, Van Hulle CA, Waldman ID, et al. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gerhardt S. Why love matters: How affection shapes a baby's brain. Routledge; 2014. [DOI] [PubMed] [Google Scholar]
- 54. Cirulli F, Berry A, Alleva E. Early disruption of the mother–infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev. 2003;27(1‐2):73‐82. [DOI] [PubMed] [Google Scholar]
- 55. Goszczyńska E, Knol‐Michałowska K, Petrykowska A. How do pregnant women justify smoking? A qualitative study with implications for nurses' and midwives' anti‐tobacco interventions. J Adv Nurs. 2016;72(7):1567‐1578. [DOI] [PubMed] [Google Scholar]
- 56. Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA, Respass J. Prenatal tobacco exposure: developmental outcomes in the neonatal period. Dev Psychol. 2011;47(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Godding V, Bonnier C, Fiasse L, et al. Does in utero exposure to heavy maternal smoking induce nicotine withdrawal symptoms in neonates? Pediatr Res. 2004;55(4):645. [DOI] [PubMed] [Google Scholar]
- 58. King E, Campbell A, Belger A, Grewen K. Prenatal nicotine exposure disrupts infant neural markers of orienting. Nicotine Tob Res. 2017;20(7):897‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wiebe SA, Fang H, Johnson C, James KE, Espy KA. Determining the impact of prenatal tobacco exposure on self‐regulation at 6 months. Dev Psychol. 2014;50(6):1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


