Abstract
In recent years, the influence of alpha (7–13 Hz) phase on visual processing has received a lot of attention. Magneto‐/encephalography (M/EEG) studies showed that alpha phase indexes visual excitability and task performance. Studies with transcranial alternating current stimulation (tACS) aim to modulate oscillations and causally impact task performance. Here, we applied right occipital tACS (O2 location) to assess the functional role of alpha phase in a series of experiments. We presented visual stimuli at different pre‐determined, experimentally controlled, phases of the entraining tACS signal, hypothesizing that this should result in an oscillatory pattern of visual performance in specifically left hemifield detection tasks. In experiment 1, we applied 10 Hz tACS and used separate psychophysical staircases for six equidistant tACS‐phase conditions, obtaining contrast thresholds for detection of visual gratings in left or right hemifield. In experiments 2 and 3, tACS was at EEG‐based individual peak alpha frequency. In experiment 2, we measured detection rates for gratings with (pseudo‐)fixed contrast. In experiment 3, participants detected brief luminance changes in a custom‐built LED device, at eight equidistant alpha phases. In none of the experiments did the primary outcome measure over phase conditions consistently reflect a one‐cycle sinusoid. However, post hoc analyses of reaction times (RT) suggested that tACS alpha phase did modulate RT for specifically left hemifield targets in both experiments 1 and 2 (not measured in experiment 3). This observation requires future confirmation, but is in line with the idea that alpha phase causally gates visual inputs through cortical excitability modulation.
Keywords: alignment, alpha, entrainment, null results, oscillations, phase
In a series of experiments, visual stimuli were presented at pre‐determined phases of an alpha‐frequency tACS signal applied to right occipital cortex. Curve‐fitting analyses revealed no effects on visual detection or thresholds, but possible post hoc effects on reaction times in left visual field.
Abbreviations
- 2AFC
2‐alternative forced choice
- cm
centimeters
- DAC
digital–analog converter
- DVA
degrees visual angle
- EEG
electroencephalography
- Hz
hertz
- IAF
individual alpha frequency
- ITI
inter‐stimulus interval
- mA
milliampere
- MEG
magnetoencephalography
- Ms
milliseconds
- NIBS
non‐invasive brain stimulation
- RT
reaction time
- tACS
transcranial alternating current stimulation
- TMS
transcranial magnetic stimulation
1. INTRODUCTION
The visual hierarchy is one of the most extensively investigated systems in the human brain, but its communication mechanisms remain unclear. In recent years, studies have increasingly focused on the role of oscillatory mechanisms in successful visual processing (e.g., Gallotto, Sack, Schuhmann, & de Graaf, 2017). Oscillations can be described in terms of frequency, amplitude and phase. In humans, magneto‐ or electroencephalography (M/EEG) can be used to measure such oscillatory activity, arising from synchronized neuronal ensembles (Berger, 1929).
Neuronal activity measured at electrodes positioned over parieto‐occipital cortex oscillating at alpha frequency (7–13 Hz) seems particularly important for vision and visual attention (e.g., Klimesch, Sauseng, & Hanslmayr, 2007; Mathewson et al., 2011). The power of posterior alpha activity decreases when opening the eyes, allowing processing of visual inputs (Berger, 1929; de Graaf, Duecker, Stankevich, Oever, & Sack, 2017). Alpha power decreases in occipito‐parietal cortex contralateral to the attended hemifield (Kelly, Lalor, Reilly, & Foxe, 2006; Romei, Brodbeck, et al., 2008; Sauseng et al., 2005; Thut, Nietzel, Brandt, & Pascual‐Leone, 2006; Worden, Foxe, Wang, & Simpson, 2000). Furthermore, alpha power relates to visual excitability: phosphenes induced by occipital pulses of transcranial magnetic stimulation (TMS) require lower stimulation intensity in participants with lower levels of resting‐state alpha power (Romei, Rihs, Brodbeck, & Thut, 2008), and on trials with lower alpha activity right before TMS pulses (Romei, Brodbeck, et al., 2008). Visual stimuli also are more readily detected on trials with lower posterior alpha power (Lange, Oostenveld, & Fries, 2013; van Dijk, Schoffelen, Oostenveld, & Jensen, 2008).
As is the case for alpha power, there is concrete evidence that the phase of ongoing alpha oscillations affects visual processing (Callaway & Yeager, 1960; Dustman & Beck, 1965). Hits (i.e., detection) or misses of flashes of light at luminance threshold were associated with different pre‐stimulus alpha–theta phase distributions (Busch, Dubois, & VanRullen, 2009). Alpha phase not only correlates with visual detection but also correlates with discrimination, for instance in visual crowding (Ronconi & Marotti, 2017) and temporal processing tasks (Ronconi, Busch, & Melcher, 2018). Mathewson, Gratton, Fabiani, Beck, and Ro (2009) showed in a metacontrast masking paradigm that posterior alpha phase could predict both visual task performance and EEG activity elicited by the visual stimulus. TMS‐induced phosphene perception also depends on alpha phase, suggesting that alpha phase indexes visual excitability (Dugué, Marque, & VanRullen, 2011). TMS pulses prior to visual stimuli can abolish their perception, possibly through effects on alpha oscillations (de Graaf, Duecker, Fernholz, & Sack, 2015; de Graaf, Koivisto, Jacobs, & Sack, 2014; Jacobs, de Graaf, & Sack, 2014).
Correlational studies as discussed above are highly informative, but also constrained. Assigning trials to power or phase bins post hoc, based on naturally occurring oscillations, limits which brain regions, frequencies or outcome measures we can evaluate (ten Oever et al., 2016). Bringing power and/or phase of oscillations in specific brain regions under experimental control would enable additional research questions. Moreover, it has been argued that turning such oscillatory measures into independent variables provides solid ground for an evaluation of their causal role in particular functions (Herrmann, Strüber, Helfrich, & Engel, 2016). Such experimental control can be achieved through rhythmic sensory (de Graaf et al., 2013; Mathewson, Fabiani, Gratton, Beck, & Lleras, 2010; Mathewson et al., 2012) or rhythmic non‐invasive brain stimulation (Thut, Schyns, & Gross, 2011). Phase alignment and amplification of neuronal oscillations by an external oscillator have been called entrainment (Thut, Schyns, et al., 2011).
Entrainment of alpha oscillations has been achieved with bursts of rhythmic TMS pulses or sustained transcranial alternating current stimulation (tACS). Alpha TMS bursts have demonstrable effects on visual task performance (Jaegle & Ro, 2014; Romei, Gross, & Thut, 2010) and local alpha power measured by EEG (Thut, Veniero, et al., 2011). Jaegle and Ro (2014) showed that visual target processing was affected by preceding parietal alpha TMS bursts in a time‐specific manner, in line with a causal role of alpha phase. A landmark study by Helfrich et al. (2014) applied parieto‐occipital alpha tACS concurrently with EEG and reported that (i) neuronal alpha oscillations were entrained, and (ii) alpha phase modulated visual performance.
This converging evidence suggests that both power and phase of posterior alpha oscillations are causally relevant for successful detection of visual targets. Specifically relevant for the study of alpha phase, we recently developed experimental methodology (ten Oever et al., 2016) that not only allows full experimental control over tACS stimulation, but also sub‐millisecond precise time‐locked presentation of (multi‐modal) sensory or magnetic stimuli to participants. Aside from other benefits, this setup allows tACS‐phase‐based stimulus presentation, which turns oscillatory phase into an independent variable. In the current series of experiments, we took advantage of this implementation to present visual gratings (experiments 1/2) or LED luminance changes (experiment 3) at pre‐determined phases of tACS administered to right occipital cortex. If tACS successfully entrained (phase‐aligned, possibly amplified) posterior neuronal oscillations, tACS alpha phase should correspond to neuronal alpha phase. This allowed us, for example, to determine contrast detection thresholds for stimuli presented at each tACS phase, separately for the right and left visual hemifields, with psychophysical staircases running in parallel for different phase conditions. This, specifically, is not possible with correlational post hoc phase binning studies, as it requires the phase on each trial to be known a priori, and opens up a wide range of new studies and more precise questions to ask.
Yet, looking ahead, we failed to find consistent evidence for a causal role of alpha phase on left hemifield contrast thresholds, or hit rates, across several attempts. Post hoc analyses did provide some support for alpha phase effects on reaction times, a secondary outcome measure recorded in experiments 1 and 2 (not experiment 3), particularly in left hemifield, across several alternative analyses. Future studies can build on the current work to further explore this approach and its findings.
2. MATERIALS AND METHODS
We performed three related experiments (exp 1, exp 2 and exp 3), for which we indicate under each header how they differ in procedure and parameters.
2.1. Participants
This series of experiments included 39 measurements in total. Fourteen participants including two authors (T.G. and S.O.) were tested in exp 1, but two were excluded prior to main analyses due to problems with hardware or task performance. Ten participants including one author (S.B.) were tested in exp 2 (one participant participated in both exp 1 and exp 2). Fifteen participants were included in exp 3. All participants had normal or corrected‐to‐normal vision, were screened for tACS safety and provided written informed consent. The experimental procedures were approved by the local ethics committee.
2.2. Transcranial alternating current stimulation and controlling equipment
In all experiments, tACS was applied using a small (3 × 3 cm) electrode applied over right occipital cortex (O2 position in the international 10–20 coordinate system) and a large (5 × 7 cm) reference electrode applied over vertex (Cz position) with anterior–posterior orientation for the longer side.
In experiment 1, peak‐to‐peak amplitude of stimulation was determined per participant. Before the main experiment, we stimulated participants briefly with the montage, with increasing stimulation intensity, asking them each time to indicate what they found comfortable and whether they perceived phosphenes to such an extent that they might interfere with the processing of visual stimuli. This resulted in a range of stimulation intensities from 0.8 to 2 mA peak to peak, with twelve out of fourteen participants stimulated at 1.2 mA or higher and an overall mean intensity of 1.5 mA. At the occipital electrode, this constitutes a mean current density of 1.67 A/m2 under the electrode.
We used the same electrode montage for tACS across all three experiments. We hypothesized that this montage should affect specifically right occipital cortex strongly, limiting effects on left hemisphere, and with relatively diffuse currents to and around the large Cz electrode, limiting modulation of brain activity there. We used the freely available SimNIBS package (Thielscher, Antunes, & Saturnino, 2015) to evaluate these predictions and visualize the modeled distribution of induced electric fields across a default brain anatomy included in the package. The resulting color map (Figure 1d) suggests that the relative distribution of normalized electric field (normE: E/Emax) was indeed centered on and quite limited to right occipital cortex. Relevant caveats are that (a) we did not obtain individual anatomical scans to allow subject‐level modeling, and (b) this visualization depicts the relative “stimulation strength” across the anatomy, it cannot indicate where or whether the stimulation was sufficiently effective to successfully modulate brain activity. In this modeling, the current intensity was set to 1.5 mA peak‐to‐peak amplitude.
Figure 1.
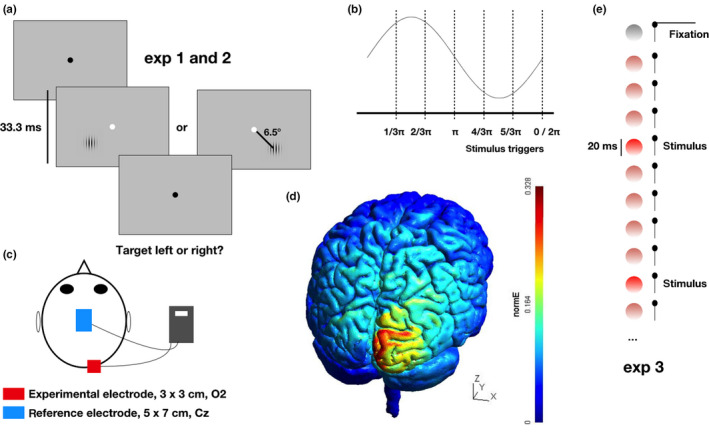
Experimental design and tasks. (a) In experiments 1 and 2, participants fixated on a central black dot. Stimuli were sinusoidal gratings of calibrated contrast, presented either lower left or right of fixation per trial. Location was uncued, the fixation dot brightened to prompt a 2‐alternative forced‐choice response about target location. (b) In all experiments, stimuli were triggered in pre‐determined phases of the ongoing tACS signal. Shown are the phase conditions for experiments 1 and 2; six equidistant phases spanning one cycle. In experiment 3, there were eight equidistant phase conditions. (c) Participants received focal tACS to right occipital cortex (O2), with a non‐focal reference electrode over vertex (Cz). (d) Using the SimNIBS package (Thielscher et al., 2015) and a default anatomy, modeling of our montage (for 1.5 mA peak to peak) resulted in relatively focal right hemispheric normalized electric fields (normE = E/E max). (e) In experiment 3, participants fixated a white Q‐tip positioned to the upper right of an LED stimulus covered by a ping‐pong ball to enlarge and diffuse the stimulus. The LED turned on to signify task start, and the LED would briefly decrease in luminance by an individually calibrated amount for 20 ms several times before turning dark again, signifying a short break. Participants responded to perceived luminance changes [Colour figure can be viewed at wileyonlinelibrary.com]
Transcranial alternating current stimulation was ramped up and down over 10 s. Stimulation duration was approximately 19 min for experiments 1/2, 20 min for experiment 3. tACS frequency was 10 Hertz (Hz) for exp 1, and at individual peak alpha frequency for exp 2 and exp 3 (see below for determination). Also in exp 2/3, tACS was briefly applied prior to the experiment, to test tolerance for somatosensory experience. We then also checked with participants that any potential phosphenes (visual experiences caused by tACS) did not occur in task‐relevant parts of the visual field. In these experiments, peak‐to‐peak amplitude was set to 1.5 mA by default, though if stimulation was deemed too uncomfortable or phosphenes overlapped with target locations, intensity was reduced to 1 mA peak to peak, which occurred once in exp 2.
Transcranial alternating current stimulation was remotely controlled, using the Remote option on NeuroConn DC‐STIMULATOR PLUS (neuroConn, GmbH, Ilmenau, Germany). We did not correct for any minor individual DC offset, which can be introduced when using Remote tACS. Electrodes were attached using Ten20 conductive neurodiagnostic electrode gel (Weaver and Company, Aurora, Colorado, USA). We previously (ten Oever et al., 2016) described our experimental setup, for which we summarize the procedures and implementation below. It involves source files created in MATLAB (TheMathWorks Inc., Natick, Massachusetts, USA), loaded into custom software DataStreamer (Oever et al., 2016). TACS signal and stimulus triggering pulses were fed through a digital–analog converter (DAC) from National Instruments (Austin, TX, USA). A standard BSC cable connected the DAC to the tACS stimulation device. The resolution of the tACS waveform in the source files was 6000 Hz in experiments 1/ 2 and 4,000 Hz in experiment 3. Stimulus‐triggering pulses consisted of digital values communicated by DataStreamer to the DAC through a parallel port connection. The DAC connected to the parallel port of a stimulus PC, running PsychToolbox (Brainard, 1997) in MATLAB and reading port activity to detect incoming triggering pulses. The pulses triggered presentation of visual stimuli on a standard LCD monitor with stimulus parameters depending on pulse values, in exp 1 and exp 2. In exp 3, pulses triggered visual stimuli not on the computer monitor, but on a custom‐built LED device connected to a parallel port of the stimulus PC.
2.3. Stimuli and tasks: experiments 1 and 2
In exp 1 and exp 2, visual stimuli were circular gratings presented on a gray background (~54 cd/m2) on the gamma‐corrected display. Participants were seated 57 cm from the display, with head fixated by the use of a chin rest. Vertical gratings, 1.5° visual angle (DVA) in diameter, presented diagonally to either lower left (left hemifield) or lower right (right hemifield) at 6.5 DVA eccentricity. Spatial frequency was 2 cycles/degree, phase was randomized each trial, and edges were faded with Gaussian blur.
The 2‐alternative forced‐choice task of participants was always to indicate, on each trial, whether a grating had been presented in the left or right hemifield with button presses on a keyboard. Per hemifield, detection rate (proportion correct) was calculated per tACS‐phase condition. As gratings were presented at peri‐threshold contrast, it was important that participants were prompted to respond, also in trials where targets were missed. A black central fixation dot, otherwise presented continuously on screen, increased in brightness for 33.3 ms (“flashed”) simultaneously with presentation of the target grating. This cue was identical across all conditions of both experiments.
In experiment 1, the contrast of visual gratings was variable across trials. In fact, separately for left and right hemifield, for each of 6 tACS‐phase conditions (see Figure 1 and below), we ran a psychophysical staircase to determine the required contrast for 80% detection rate for that hemifield/phase condition. The dependent variable across conditions was therefore the contrast threshold. The staircases used the Quest (Watson & Pelli, 1983) functionality in PsychToolbox, a Bayesian staircase algorithm that can suggest per trial the optimal test value and converges on final estimates based on the entire history of test values and responses in a pre‐determined number of test trials (40 trials per staircase in our experiment). We supplied Quest with following parameters: beta = 3.5, gamma = 0.5, delta = 0.01, tGuess = 1 and tSD = 1.
In experiment 2, the contrasts of visual gratings were pseudo‐fixed over trials. Quest staircases, separately for left and right hemifield, determined individually calibrated contrasts to achieve 75% detection rates, prior to the main experiment. This was slightly lower than the 80% aim performance in experiment 1 to allow for performance increases over the course of the session. After all, the 80% was to be achieved by the end of sessions in experiment 1, while the 75% performance was established at the start of sessions in experiment 2. These contrasts were then fixed for the main experiment, in principle, and the dependent variable was accuracy over hemifield and tACS‐phase conditions. However, per hemifield, but independently of tACS‐phase conditions, the experiment program did keep track of task performance over time. As we were interested in a potentially oscillating pattern of our outcome measures over tACS‐phase conditions, it was important that accuracy overall would not reach ceiling or floor levels. Thus, if accuracy in the most recent 5 trials reached 60% (approaching floor) or 90% (approaching ceiling), contrast was increased or decreased by 10% of its previous value, respectively.
2.4. Stimuli and tasks: experiment 3
In experiment 3, visual stimulation was an LED, briefly changing luminance. Participants placed their heads in a chin rest in a dark environment (a dark cloth placed over their heads in a laboratory with lights off), and the LED device was positioned 35 cm in front of them. On top of the LED device was a white stick of cotton swab (Q‐tip), to serve as a fixation point to the upper right of the LED. The LED stimulus was enlarged and diffused by placing a punctured white ping‐pong ball over it, to help avoid fading effects. All this resulted in a red visual stimulus with a diameter of approximately 4 cm, at a viewing distance of approximately 35 cm. From the view of the participant, the cotton swab fixation point was approximately 0.5 cm above the top right edge of the ping‐pong ball, placing the red target stimulus in the periphery. Participants clicked a left mouse button whenever they perceived a brief change in luminance (a “flicker”). The LED was in principle always on during trials, and it regularly turned off to indicate breaks. Trial events were not cued in this experiment, in contrast to experiments 1/2. This experiment was therefore a signal detection task, rather than 2‐alternative forced‐choice task. A Quest staircase prior to the main experiment determined the decrease in LED luminance required to obtain a 45% detection rate. This luminance change was then pseudo‐fixed for the main experiment. Performance on the most recent 10 trials was monitored by the experiment program, and the luminance change was increased or decreased by a factor depending on the amount of deviation from the target performance range (0.3–0.7 proportion of stimuli detected). The dependent variable for this experiment was thus detection rate of luminance changes, defined as the proportion of trials per tACS‐phase condition on which participants pressed the button within 1.4 s of an actual luminance change. This response window duration was somewhat arbitrarily chosen and implemented in the experiment code. No reaction times were recorded: per target presentation, only a “1” was logged if a response was supplied within that response window post‐target (and otherwise a “0”).
2.5. tACS‐phase conditions and phase‐locked visual presentation
Of course, the core methodology across all experiments was the presentation of stimuli at predefined phases of the entraining tACS signal. This was achieved, as described previously (ten Oever et al., 2016) and summarized above, by generating source files containing both the tACS stimulation and the desired timing of stimuli in relation to that tACS signal. Concretely, these source files contained values oscillating between −1 and +1, scaled by DataStreamer software to the desired tACS intensity, at a particular sampling frequency. In a secondary timeline in the source files, “pulses” were coded to indicate the timing (by their position in the timeline) and parameters (by their numerical value) of visual stimuli.
In experiments 1/2, per hemifield there were 6 tACS‐phase conditions. This number relates to the refresh rate of our display, which is 60Hz and therefore can present six frames per 100 milliseconds (ms). Since one cycle of 10 Hz lasts 100 ms, our “sampling resolution” using this display was limited to six phases of a 100 ms cycle. In the source files, there were therefore 12 possible “pulse values.” One through 6 indicated that a stimulus should be presented in the left hemifield, and 7 through 12 indicated a stimulus should be presented in the right hemifield. Taking 1 through 6 as our example, each of these values was presented precisely time‐locked to always the same phase of tACS, spanning one full cycle. Thus, in radians, tACS phase “1” was always at 1/6*2pi, “2” was always at 2/6*2pi, etc. In experiment 1, each numerical value, directly reflecting one tACS‐phase condition, was associated with its own psychophysical staircase. At the end of each trial, the participant response was processed, the next contrast value to be tested was determined by Quest, and the visual grating was prepared for the next trial of that particular phase condition and hemifield.
Between trials, the experiment program was continuously scanning for incoming inputs. As soon as a triggering pulse was received, the program would as quickly as possible display the associated visual grating on screen along with the central response cue. This presentation of gratings whenever a triggering pulse was received was inevitably not instant. Moreover, with a 60 Hz display, even if computational processing was instant, the time from incoming trigger to actual display would be anywhere between 0 and 16.7 ms, if the stimulus were presented at the first next available frame (screen refresh). Since the internal clocks of the PC running DataStreamer and the PC presenting visual stimuli were likely not perfectly synchronized, running an experiment like this over tens of minutes would also mean that these delays were not constant. If the first next frame was “missed,” the delay could be slightly longer. With a period of on average 100 ms, and a corresponding separation of tACS‐phase conditions of 16.7 ms, these delay variations go toward measurement noise and should be kept in mind. This is, however, not the case in exp 3, in which we used an LED device for visual stimulation explicitly to avoid such limitations. Note that, in exp 2, the use of individual peak alpha frequencies for tACS introduces an additional limitation: Though triggering pulses were sent at 6 equidistant phases of the tACS signal, the display and thereby actual visual presentations were still constrained to the same frame rate of 60 Hz.
We did quantify and analyze these delays based on the timestamps provided by the stimulation software. For the purposes of this study, absolute delays are irrelevant since the analyses and research questions fully apply to between phase‐condition differences. Most important are therefore (a) the within‐subject consistency of delays between condition phase angle (the incoming “stimulus trigger”) to actual stimulus onset from trial to trial, and (b) the consistency of these delays from phase condition to phase condition. To estimate the trial‐by‐trial consistency of these delays, we calculated the percentile range containing 95% of all recorded delays in milliseconds (2.5th to 97.5th percentile). This range estimator was calculated per participant and per phase condition (6 for left hemifield, 6 for right hemifield), separately for experiment 1 and experiment 2. In Tables S1‐S3, we report all these values per participant per experimental condition, as well as on group level per condition, but they were all similar. The mean [standard deviation] of 95% range values in experiment 1 across all phase/hemifield conditions was 15.8 ms [1.7 ms]. Looking at this range estimator across participants but per condition (6 phase × 2 hemifield), this seemed consistent over conditions; lowest median range estimator across participants was 15.2 ms, and highest median range estimator was 15.9 ms. Note that these values are based on timestamps provided by the presentation software, not based on luminance measurements on the monitor. Many more details are provided in supplementary material.
It is an open question to what extent our null findings, or statistical strength of post hoc positive findings, are attributable to these sources of measurement noise. Future studies could employ LED devices (as here in experiment 3) and measure multiple outcome variables (here we did not record reaction times in experiment 3). Alternatively, monitors with higher frame rate should reduce the range of this source of measurement noise.
2.6. Experiment parameters and procedures
2.6.1. Experiment 1
Two visual hemifields and six phase conditions resulted in a total of 12 condition cells in a 2 × 6 design. Per cell, we collected 40 trials using Quest staircases. Using the QuestMean function, we extracted from the staircase performance in each condition cell a final estimate of the contrast required for 80% task performance. Visual stimulus duration was 33.3 ms, inter‐trial duration was jittered around 2 s, and the experiment included 5 breaks of 15 s.
In experimental sessions, participants first received explanations of the tasks and experimental procedures. They performed calibration measurements using Quest staircases, serving also as task practice. tACS electrodes were applied, tACS was applied at 10 Hz, and participant could report on tolerance and phosphene perception. Then, the tACS device was set to the Remote option, and the experiment program was started, which triggered DataStreamer software to start reading out its source file, containing the tACS signal and the tACS‐phase‐locked stimulus triggers.
2.6.2. Experiment 2
The same 2 × 6 design as in exp 1 was implemented with 40 trials per condition cell. Visual stimulus duration was 33.3 ms, inter‐trial duration was jittered around 2 s, and breaks were offered approximately every 3 min. As above, participants received explanations of tasks and procedure, were screened for tACS safety and provided written informed consent. Participants first performed two or three calibration measurements, depending on consistency of outcome, which were Quest staircases with parameters beta = 3.5, gamma = 0.5, delta = 0.01, tGuess = 0.8 and tSD = 1. Using the QuestMean function, each staircase yielded a contrast level for 75% correct detection, and contrast threshold results for repeated and included staircases were averaged.
Next, we determined individual peak alpha frequency (IAF). We applied single EEG electrodes to positions P4, right mastoid (reference), and Fz (ground), of the international 10–20 coordinate system. Participants closed their eyes and relaxed for 150 s while we recorded EEG. A fast Fourier transform using the FieldTrip (Oostenveld, Fries, Maris, & Schoffelen, 2011; Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, The Netherlands) ft_freqanalysis.m function yielded powerspectra for 5‐s epochs which were then averaged. We determined the local maximum of the resulting spectrum within the alpha‐band: window 7–13 Hz. The frequency corresponding to this local maximum was taken as IAF and used to build the tACS source file. The main experiment proceeded as described for exp 1. In this experiment and experiment 3, a coding error resulted in identical trial order for most participants. This means that, although the order of trials (conditions) was randomized and unpredictable for participants, it was not different between participants.
2.6.3. Experiment 3
As the visual stimulation device here was a custom‐built LED device, there was only one visual field (left) and we increased the number of tACS‐phase conditions to eight. Per phase condition, we collected 30 trials. Trials had no clear beginning or end for participants. If the LED turned on, participants knew to pay attention to the stimulus and report any perceived luminance changes. Responses could reflect false alarms or hits. To constrain stimulus regularity/predictability, we created inter‐stimulus intervals (ITI) based on a gamma distribution: Each ITI was 2 + g seconds, where g was a randomly selected value from a gamma distribution with shape parameter 1 and scale parameter 1.3. Mean inter‐stimulus interval was therefore 3.3 s, but between any two trials, the interval could be up to 15 s. Breaks were indicated by the LED turning off and were given every 25 trials for 20 s. A longer break of 120 s, in which we removed the black cloth covering the participant and stimulus display, and turned on the lab lights, occurred after 129 trials. Lastly, to help prevent fading and allow participants a brief moment to relax and blink their eyes, the LED light turned off for 0.8 s every 5 trials.
Otherwise, procedures were similar to experiments 1/2, and individual alpha frequency (IAF) was determined as in exp 2. In this experiment, three Quest staircases were run to determine the LED luminance change yielding 45% detection of the stimulus. This luminance change was used as visual stimulus for the main experiment, but adapted as dictated by performance across all conditions over the course of the experiment as mentioned above.
2.7. Preprocessing
In experiments 1 and 2, trials on which participants pressed an incorrect key (not corresponding to either of the response options) or pressed a key too late (1.4 s allotted for response) were excluded (in the case of staircases in experiment 1, these trials did not contribute to the online estimation procedure). Post hoc, trials with response times below 200 ms were also excluded. In experiment 1, staircase outcomes (based on QuestMean.m function) were recomputed after removal of these trials, by rerunning the staircases with simulated responses corresponding to actual responses from the remaining, included trials.
2.8. Analyses and statistical tests
The outcome measures differed over experiments (exp 1: contrast thresholds, exp 2: accuracy, exp 3: detection rate), but the analyses were largely identical. After all, whichever outcome measure we used, the hypothesis was always that this outcome measure would display a one‐cycle sinusoid over tACS‐phase conditions. This is the consequence of our hypothesis that visual task performance should oscillate along with neuronal oscillations, which should oscillate along with tACS. The applied statistical analyses were therefore designed specifically to assess whether (a) tACS‐phase effects occurred, and particularly whether (b) a one‐cycle sinus matched the pattern of behavioral outcomes over the sampled phase conditions.
On different levels (participant level, group level), we fitted sinuses to the 6 (exp 1/exp 2) or 8 (exp 3) datapoints based on minimization of squared errors. These sinuses had free amplitude and phase, but fixed frequency (exactly one cycle across the datapoints). The goodness of fit would be reflected in R‐squared; variance in datapoints explained by the sinusoid fit. Inspired by previous reports (Fiebelkorn et al., 2011; ten Oever & Sack, 2015; Schilberg et al., 2018), we multiplied R‐squared by the variance of the best fitting sinusoid, to obtain relevance values. As we had so few sample points, we calculated the variance of the best fitting sinusoid not only based on the sinusoid values at the sample points, but calculated population variance of 100 equidistant values on one full cycle of the best fitting sinusoid. This variance measure of the full sinusoid was multiplied with R‐squared, which was calculated as 1 – (SSM/SST), where SST was the sum of squared differences between observed data at sample points and their mean, and SSM was the sum of squared differences between observed data at sample points and the sinusoid values at those sample points. The resulting hybrid measure of relevance value should reflect both the goodness of fit (R‐squared) and the extent of modulation of performance by tACS, both of which are of interest in the current context. Note that in the Results section and figures, R‐squared is often reported, as a more intuitive outcome measure, but the associated p‐values are based on permutation tests of these relevance values, not R‐squared values.
We used permutation tests to determine the statistical significance of obtained relevance values. For each result we wanted to statistically assess, we would build a null distribution against which to test it. This null distribution was created by repeating exactly the same processing steps on the same data, but after shuffling individual trial labels. In experiment 1, as the core outcome measure was the result of a Quest staircase procedure (using QuestMean.m function), each shuffling of trial labels was followed by a recomputation of the outcome of the Quest staircase algorithm, through simulation of the responses based on (shuffled) real responses. These steps were always taken separately per participant and in exp 1/2 per visual hemifield, so only the phase‐condition labels of trials were shuffled. Two thousand iterations of shuffling and re‐calculation of results (e.g., individual accuracy per condition, staircase outcome, subsequent fitting‐based relevance value) led to a distribution of results. The P‐values we report are the proportion of permuted results (i.e., null distribution) larger than the actually obtained result. We consider outcomes falling in the last 0.05 of the null distribution to be statistically significant.
These procedures apply to all the following analyses. On the group level, we performed three different analyses. (1) We phase‐aligned, Z‐scored, and then averaged the individual participants’ outcome measures of phase conditions into a group result pattern. We then performed the sinusoid curve‐fitting analysis on this group result. Significance of the resulting fit (relevance value) was evaluated by repeating this entire analysis 2,000 times on the data with shuffled trial labels (permutation test as described above). For various reasons (e.g., retino‐cortical transmission time, individual visual cortical anatomy), it is not a priori expected that, even in the case of successful tACS‐phase modulation of visual processing, individual results should phase‐align. Therefore, we used the maxima in individual outcomes over phase conditions (i.e., the phase conditions with absolute highest task performance) to phase‐shift individual results. Importantly, we left out these peak values, used for the phase alignment, from the group analysis.
(2) To allow for the possibility that tACS phase could modulate performance, just not in a sinusoidal fashion, we also tested after phase alignment the group average of the “up‐phase” conditions (as phase condition 2 was the “peak” after phase alignment and therefore removed: up‐phase condition was the group average of phases 1 and 3) against the group average of the “down‐phase” conditions (group average of phases 4, 5 and 6). (In exp 3, the analogous contrasted phases were 1, 3, 4 against 5, 6, 7, 8.) This contrast was tested in a permutation test on the up‐phase minus down‐phase means: Trial shuffling the phase condition labels 2000 times and always repeating the full analysis procedure up to calculation of a group up‐phase average minus group down‐phase average. (3) These analyses effectively constitute fixed‐effects analyses. Therefore, we based a final group analysis on the results of individual curve‐fitting analyses and associated permutation tests. For each participant, the curve‐fitting procedure and individual permutation test assessed to what extent the individual pattern of performance over phase conditions statistically significantly matched a sinusoid. The resulting p‐value per participant, per visual field location and per dependent variable (see below) was converted to a Z‐score. The resulting vector of Z‐scores was tested against 0 in a one‐sample t test. A significant deviation of the mean Z‐score from 0 should indicate that, over participants, the sinusoid curves consistently explained more data than expected by chance, even if effects were too small on the individual subject level to reach significance.
Importantly, a priori we planned these analyses for the following dependent variables: contrast detection thresholds in exp 1, accuracy (proportion correct) in exp 2 and hit rate in exp 3. However, given the inconsistent and null results for these dependent variables (see Results), we post hoc decided also to perform and report the same analyses for the dependent variable of reaction times, which were recorded in exp 1 and exp 2. On the subject level and condition level, mean reaction times were estimated based only on correct trials. As will be clear below, analyses did provide some support for effects of tACS phase on reaction times. This is not unexpected, but it should be kept in mind that these analyses were secondary and post hoc.
On a final note, we performed some final additional exploratory analyses, detailed and reported in Supplementary Material. For specifically experiment 1, we performed all group analyses also on two subsamples of the included participants based on a median split of the tACS intensity, as in this experiment the intensities varied. This is described in detail in Supplementary Methods and Results. For all three experiments, we repeated the group analyses without Z‐scoring individual performance patterns prior to group analysis. Lastly, for all three experiments, we used a fast Fourier transform (FFT) approach to quantify single‐cycle oscillations, instead of a curve‐fitting approach with relevance values.
3. RESULTS
In experiment 1, we tested the modulation of contrast thresholds for gratings in lower left or lower right visual hemifields, when presenting these gratings at six different phases of 10Hz tACS administered to right occipital cortex (location O2). In experiment 2, we tested modulation of grating detection performance (hit rates) using pseudo‐fixed thresholds, by different phases of tACS at individual peak alpha frequencies (IAF). In experiment 3, we tested modulation of LED luminance change detection by eight different phases of IAF tACS. We present the results sequentially. We additionally report the outcomes when performing the same analyses on reaction times in experiments 1 and 2 (not recorded in experiment 3).
3.1. Experiment 1
3.1.1. Individual results
The full pattern of individual results is shown in Figures SF1 and SF2, which present the thresholds (SF1) and mean reaction times (SF2), per hemifield, across tACS‐phase conditions. Best fitting one‐cycle sinusoids are superimposed, and figure titles provide the explained variances, as well as the P‐values based on permutation tests on the relevance values of the curve‐fitting approach (see Methods). Table 1 below also provides the variances explained by fitted sinusoids (R‐squared; contrast threshold in italics as the primary analysis, reaction time as post hoc analysis) along with p‐values resulting from permutation tests on the relevance values (R‐squared multiplied by amplitude of best fitting sinusoid, see Methods). Conditions in which the relevance value was statistically significant (uncorrected) are indicated in bold.
Table 1.
Individual results. Explained variance (R‐squared) with p‐value resulting from permutation tests on relevance values in parentheses. Threshold analysis was the main analysis (italic); reaction time analysis was post hoc. Bold cells are statistically significant (uncorrected)
| Left visual field | Right visual field | |||
|---|---|---|---|---|
| Threshold | RT | Threshold | RT | |
| 1 | 0.05 (0.941) | 0.45 (0.608) | 0.49 (0.619) | 0.10 (0.795) |
| 2 | 0.10 (0.864) | 0.28 (0.230) | 0.74 (0.239) | 0.59 (0.007) |
| 3 | 0.05 (0.950) | 0.27 (0.516) | 0.18 (0.752) | 0.42 (0.110) |
| 4 | 0.12 (0.586) | 0.84 (0.013) | 0.17 (0.783) | 0.00 (0. 996) |
| 5 | 0.32 (0.571) | 0.64 (0.116) | 0.60 (0.607) | 0.53 (0.120) |
| 6 | 0.41 (0.152) | 0.60 (0.352) | 0.54 (0.478) | 0.09 (0.751) |
| 7 | 0.22 (0.861) | 0.64 (0.007) | 0.39 (0.006) | 0.59 (0.013) |
| 8 | 0.28 (0.552) | 0.21 (0.414) | 0.42 (0.010) | 0.47 (0.094) |
| 9 | 0.44 (0.777) | 0.69 (0.056) | 0.47 (0.273) | 0.25 (0.763) |
| 10 | 0.42 (0.357) | 0.64 (0.001) | 0.20 (0.622) | 0.30 (0.608) |
| 11 | 0.21 (0.704) | 0.75 (0.028) | 0.75 (0.108) | 0.32 (0.122) |
| 12 | 0.05 (0.926) | 0.41 (0.339) | 0.64 (0.364) | 0.71 (0.000) |
Three observations are relevant in Table 1 (and SF2/SF3). Firstly, while our main hypothesis for exp 1 was that contrast thresholds should be modulated by alpha tACS phase especially in the left hemifield, not a single participant showed such modulation statistically significantly. For right hemifield targets, without multiple comparison correction two participants showed modulation that reached significance. Secondly, sinusoids often did explain a lot of variance even if not statistically significant. This is also apparent from SF2/SF3: The goodness of fit is often visually impressive. Yet, likely due to the limited number of phase conditions and the inevitable sampling of only a single oscillatory cycle, an oscillatory pattern well matched by a sinusoid can appear by chance relatively easily. This emphasizes how important it is to think critically about the appropriate statistical procedures to test the observed goodness of fits. Thirdly, our post hoc additional analysis evaluated modulation of reaction times (RT) by alpha tACS phase, and here specifically in left hemifield, there were four participants with significant modulation (plus another approaching significance), versus three in the right hemifield.
3.2. Group analyses
It is difficult to credit so many individual statistical tests or to draw generalized conclusions from them. We performed a second‐level group analysis on these individual results. Per hemifield, and separately for contrast thresholds and mean RTs, the individual p‐values were converted to Z‐scores, which were subsequently tested against zero in a one‐sided t test across participants. Essentially, this approach evaluates the extent to which the relevance values of individual participants were consistently toward the extreme (right) end of their associated permutation‐based null distributions. Thus, this test might capture consistent but small effects, too weak to be significant in individual subject statistics but meaningful across the sample. This test, however, did not reveal tACS‐phase modulation of contrast thresholds of left hemifield targets (t(11) = −2.61, p = .99), or right hemifield targets (t(11) = 1.44, p = .09). In a post hoc analysis of reaction times, it indicated that reaction times to specifically left targets might have been modulated by alpha tACS phase (left hemifield: t(11) = 3.62, p = .002, right hemifield: t(11) = 1.26, p = .12). This effect even survives Bonferroni correction for these four tests, but keep in mind that (a) the reaction time analysis was post hoc, and (b) the experiment was not designed with this analysis in mind. So we would like to replicate it in experiment 2, and in the other group analyses, we performed on these data.
In a second group analysis, we first phase‐aligned individual performance patterns, phase‐shifting each pattern based on the absolute peak in performance across the six phase conditions. These peak datapoints were then excluded from further analysis. The remaining (five) phase condition results were averaged across participants, and a once‐cycle sinusoid was fitted to the group average result. See Figure 2 for a visualization of these group results of experiments 1, 2 and 3. In these analyses, only amplitude was a free parameter, as we phase‐locked the fitted sinusoid such that its peak corresponded to the (removed) peaks of the phase‐aligned observed data. Relevance values resulting from this curve fitting were tested against a null distribution built from relevance values obtained by this exact same analysis performed on all 2000 trial‐shuffled datasets obtained when performing the individual participant permutation tests. There was no effect of tACS phase on left hemifield contrast thresholds (R 2 = 0, p = .55), and a trend for right hemifield contrast thresholds (R 2 = 0.43, p = .08). There was a trend for tACS‐phase modulation of reaction times to left hemifield targets (R 2 = 0.37, p = .0505), not right hemifield targets (R 2 = 0, p = .79).
Figure 2.
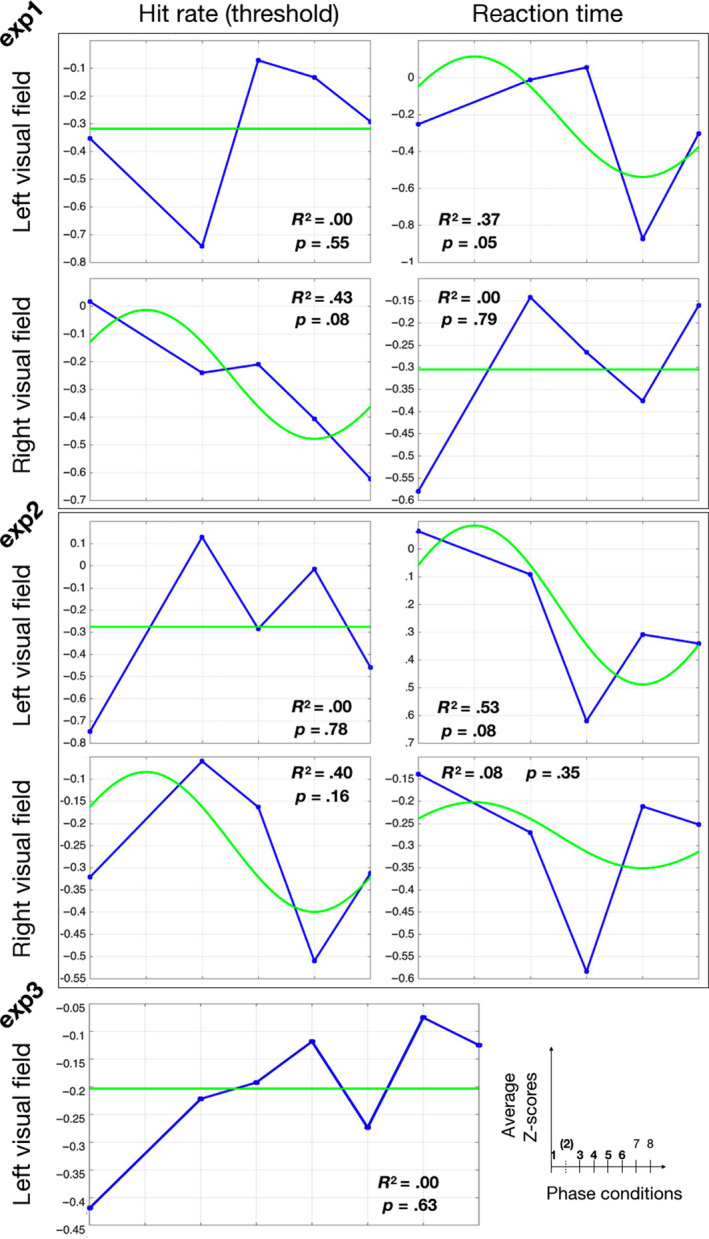
Phase‐aligned group average results. Individual observed results (contrast thresholds and mean RT in exp 1, accuracy and mean RT in exp 2, hit rate in exp 3) were Z‐scored, then phase‐shifted such that the absolute peak value was in phase slot “2” for each participant, then averaged across participants (blue lines). The group data point for phase slot “2” was left out of graphs and analysis, as it was an average of the individual datapoints used for phase alignment. tACS‐phase modulation of behavioral measures should result in a one‐cycle sinusoidal pattern over the remaining phase conditions, with its peak at phase slot 2. Thus phase‐locked best fitting sinusoids are shown in green. Above each graph, we present the goodness of fit of these sinusoidal fits, expressed by R‐squared (Rsq), and the p‐value to come out of a permutation test of the associated relevance value (a measure reflecting both the variance explained and the extent of modulation, see Methods) [Colour figure can be viewed at wileyonlinelibrary.com]
In a final analysis, based on this same phase alignment procedure, a permutation test comparison of the averaged “up‐phase” and “down‐phase” conditions evaluated phase modulation with less of a requirement on a sinusoidal pattern (see Methods). In this analysis, tACS phase nearly significantly modulated right hemifield thresholds (p = .0595), not left hemifield thresholds (p = .98) or reaction times (left: p = .13, right: p = .67).
In sum, the analyses reported here paint a somewhat inconsistent picture, with essentially no support for contrast threshold modulations in left hemifield, which was the focus of this experiment. Instead, depending on the analysis, there were indications that perhaps thresholds were modulated in right hemifield. And there was evidence that reaction times to targets in left hemifield followed a sinusoidal pattern over tACS‐phase conditions. But given the post hoc nature of some of these analyses, replications of these potential effects are desirable before conclusions are warranted.
As mentioned in Methods, and as detailed in Supplementary Methods, we repeated all group analyses for two subsamples of experiment 1 based on a median split of the tACS intensities (which varied only in this experiment). As this resulted in two small samples of only 6 participants each, no strong conclusions can be drawn. But, somewhat encouragingly, the results for the full sample seemed particularly driven by the participants stimulated with the higher tACS intensity. All results are detailed in Supplementary Results.
3.3. Experiment 2
3.3.1. Individual results
Figures SF4/SF5 present individual result accuracies (SF4) and mean reaction times (SF5), with best fitting one‐cycle sinusoids superimposed. Figure titles also provide the explained variances, and the p‐values based on permutation tests on the relevance values of the curve‐fitting approach. Table 2 also provides the variances explained by fitted sinusoids (R‐squared) for both dependent variables across conditions.
Table 2.
Individual results. Explained variance (R‐squared) with p‐value resulting from permutation tests on relevance values in parentheses. Hit rate analysis was the main analysis (italic); reaction time analysis was post hoc. Bold cells are statistically significant (uncorrected)
| Left visual field | Right visual field | |||
|---|---|---|---|---|
| Hit rate | RT | Hit rate | RT | |
| 1 | 0.64 (0.015) | 0.17 (0.622) | 0.22 (0.618) | 0.61 (0.389) |
| 2 | 0.30 (0.486) | 0.96 (0.029) | 0.39 (0.412) | 0.52 (0.226) |
| 3 | 0.37 (0.490) | 0.35 (0.504) | 0.16 (0.750) | 0.37 (0.569) |
| 4 | 0.73 (0.239) | 0.59 (0.528) | 0.40 (0.359) | 0.42 (0.270) |
| 5 | 0.41 (0.649) | 0.26 (0.713) | 0.10 (0.965) | 0.03 (0.971) |
| 6 | 0.04 (0.945) | 0.51 (0.500) | 0.56 (0.349) | 0.56 (0.198) |
| 7 | 0.69 (0.392) | 0.70 (0.162) | 0.66 (0.359) | 0.12 (0.833) |
| 8 | 0.01 (0.996) | 0.42 (0.585) | 0.02 (0.974) | 0.84 (0.142) |
| 9 | 0.20 (0.682) | 0.65 (0.101) | 0.91 (0.093) | 0.49 (0.475) |
| 10 | 0.39 (0.363) | 0.65 (0.249) | 0.56 (0.132) | 0.62 (0.391) |
3.3.2. Group results
Per hemifield, and per dependent variable, the individual P‐values were converted to Z‐scores, which were subsequently tested to be higher than zero in a second‐level, one‐sided, uncorrected t test across participants. This test did not reject the null hypothesis for hit rates (left hemifield: t(9) = −0.37, p = .64, right hemifield: t(9) = −0.27, p = .60), with a statistical trend for reaction time (RT) for left hemifield targets (left hemifield: t(9) = 1.45, p = .09, right hemifield: t(9) = 0.30, p = .38).
For the other group analyses, we first phase‐aligned and Z‐scored individual performance patterns, then removed the data points used for phase alignment and then averaged them to create a group graph, as in exp 1 (and exp 3) and as shown in Figure 2. Accuracy on targets was not modulated by tACS phase (left hemifield: R 2 = 0, p = .78, right hemifield: R 2 = 0.40, p = .16). In the post hoc analysis of reaction times, we observed a marginally significant (R 2 = 0.53, p = .08) effect of tACS phase on reaction times for left hemifield targets, but not right (R 2 = 0.08, p = .35). The permutation tests on the averaged “up‐phase” and “down‐phase” conditions (see Methods) yielded a significant effect of tACS phase on group average reaction times for left hemifield targets only (p = .047, other p’s > .05).
3.4. Experiment 3
The third experiment evaluated a luminance change detection task with superior stimulus timing control, in which we recorded only hits (and misses) to calculate a hit rate per participant per phase condition (8 phase conditions).
3.4.1. Individual results
Figure SF6 shows the individual results, the associated best fitting sinusoids and goodness of fits (R‐squared). Relevance values based on these fits were tested against individual null distributions. For no participant in the sample of 15 did a one‐cycle sinusoid explain the data significantly better than chance.
3.4.2. Group results
For a second‐level group analysis on the individual permutation results, the Z‐scores corresponding to individual p‐values were t tested against zero, after removal of one statistical outlier value (inclusion did not qualitatively change the outcome). They were not significantly different from zero (t(13) = −0.33, p = .63), hence no evidence that tACS phase modulated luminance change detection.
Individual results (hit rates over phase conditions) were Z‐scored, phase‐shifted and averaged. As shown in Figure 2, R‐squared is essentially 0 and p = .63. Also in the final group analysis, permutation testing the mean hit rate of the up‐phase (phase conditions 1, 3, 4 in Figure 2) versus the down‐phase (phase conditions 5–8) of the phase‐shifted group average resulted in no effect (p = .69).
As mentioned in Methods above, for all three experiments we performed exploratory additional analyses, repeating analyses without Z‐scoring individual performance patterns and analyses based on fast Fourier transforms instead of curve‐fitting approaches. Tables S4 and S5 present p‐values corresponding to these analyses, which may offer additional insights. Some of the positive results presented in this main Results section receive additional support.
4. DISCUSSION
In the current series of experiments, we implemented an advanced methodological setup (ten Oever et al., 2016) to test the causal relevance of right occipital alpha‐frequency tACS phase for visual processing. In experiment 1, we performed psychophysical staircases to estimate contrast thresholds for 2AFC (2‐alternative forced choice) grating detection, separately and in parallel for conditions in which gratings were triggered at six pre‐determined phases of the 10Hz tACS signal. In experiment 2, the tACS was administered at EEG‐based individual alpha frequencies and target detection performance was tested at pseudo‐fixed contrast levels. In experiment 3, a custom‐made LED device was used to test potential modulation of signal detection performance (a brief LED luminance change) occurring at eight pre‐determined individual alpha‐frequency tACS phases.
In a series of analyses, across all three experiments, no consistent tACS‐phase modulations of these core‐dependent variables could be revealed. However, we did find evidence that reaction times to targets in the left hemifield were modulated by tACS. These findings, obtained in both experiments that recorded reaction times, are of interest. But they are based on post hoc analysis and obtained in experiments that were not explicitly designed to reliably assess reaction times. For this finding, as well as potential effects on performance in the right hemifield, additional post hoc analyses reported in the supplementary material provide additional information. These post hoc analyses suggest that the positive results, at least for experiment 1, were robust across alternative analyses. Coupled with the fact that they occur in left hemifield, and particularly in high‐intensity tACS participants (experiment 1, see Supplementary Material), these are encouraging exploratory observations.
As these remain post hoc observations, we are hesitant to overextend our interpretation. But it should be noted that alpha phase effects on reaction times do match prior research. Alpha phase has been related to manual reaction times in EEG experiments (Callaway & Yeager, 1960; Dustman & Beck, 1965). This is in line with the “excitability hypothesis” (Bishop, 1933; Lindsley, 1952) that occipital alpha phase reflects the bottom‐up cortical excitability of occipital neurons. There is also evidence that frontal (high‐)alpha phase predicts saccadic reaction times (Drewes & VanRullen, 2011). For alpha power, the relation to visual efficiency has been attributed to response bias rather than sensitivity (Iemi, Chaumon, Crouzet, & Busch, 2017). In our experiment, phase effects on reaction times could reflect either of the two, though improved sensitivity might have been expected to affect the primary behavioral measures (contrast thresholds and hit rates) as well. These considerations reflect an ongoing discussion on the nature of the relation between alpha phase and visual processing. Sherman, Kanai, Seth, and VanRullen (2016), for example, found no relation between alpha phase and sensitivity, and rather suggested that (pre‐stimulus) alpha phase reflects modulation of decision threshold by prior expectations. When it comes to positive results with tACS, one should consider recent reports that some findings could be attributable to transcutaneous/somatosensory stimulation rather than direct neural effects underneath the electrode (Asamoah et al., 2019). Future studies should evaluate to what extent tACS‐phase‐specific effects on visual perception could be confounded by such indirect effects as well as peripheral phosphenes.
For our dependent variables of a priori interest, contrast thresholds, hit rates and signal detection rates, our null results in the targeted left hemifield can be explained in several ways. Firstly, perhaps tACS did not successfully phase‐align neuronal alpha oscillations, and while naturally occurring alpha phase is functionally relevant, alpha tACS phase is not (but see Helfrich et al., 2014). While alpha tACS effects on EEG alpha power have been reported repeatedly (Kasten, Dowsett, & Herrmann, 2016; Neuling, Rach, & Herrmann, 2013), it is possible that mechanisms other than phase alignment (i.e., stochastic resonance, Vossen, Gross, & Thut, 2015) underlie some of those effects. A recent study showed that the posterior alpha rhythm might not always be easily entrained by external sources, including sensory (Keitel, Benwell, Thut, & Gross, 2018). Secondly, perhaps the phase of natural alpha oscillations occurring at lower right occipital cortex is not functionally relevant for hit rate/thresholds. Using TMS, Jaegle and Ro (2014) could show that the phase of an alpha train was functionally relevant for stimulus perception when the TMS was applied over parietal cortex, but not when applied over occipital cortex. In monkeys, recent work linked alpha phase to visual detection in parietal cortex (Fiebelkorn, Pinsk, & Kastner, 2018), and even frontal cortex and thalamus (Fiebelkorn et al., 2018; Fiebelkorn, Pinsk, & Kastner, 2019). Thirdly, perhaps the problem is in the dependent variables. In the context of alpha power, Lange et al. (2013) showed that alpha power indexes enhanced cortical excitability, not improved visual perception. One might ask the same question about alpha phase, as discussed above. Contrast thresholds and hit rates might better capture visual acuity while reaction times better capture excitability. Lastly, it is of course possible that our null results are trivial; perhaps some aspects of our methodology were suboptimal to revealing tACS‐phase modulations which could have been obtained with larger samples (each of the current experiments in isolation had a small sample size) or different experimental setup, electrode montage, or design. We did validate the experimental approach itself (ten Oever et al., 2016), and recently used it to demonstrate beta‐frequency tACS‐phase effects on motor‐evoked potentials (Schilberg et al., 2018).
5. CONCLUSION
It remains difficult to interpret null results in NIBS (see de Graaf & Sack, 2011; de Graaf & Sack, 2018). The collection of null results presented here is inconclusive in our view (Level C‐B null evidence, see de Graaf & Sack, 2018). Yet they seem worthy of dissemination to guide future studies, share the advanced experimental procedures and analysis approaches, and for the sake of transparency in a growing literature of alpha power and phase studies. The post hoc positive results for tACS‐phase modulation of left hemifield reaction times are promising, and not unexpected, but should be interpreted with caution for reasons outlined above.
CONFLICT OF INTEREST
No conflict of interest to declare.
AUTHOR CONTRIBUTIONS
S.J., S.B. and A.T. contributed to design and measurements of experiments 1 through 3, respectively. S.O. contributed to methods, analyses and writing. A.S. facilitated the research, results interpretation and helped write the paper. All authors gave feedback on the paper. T.G. developed the experiments, performed the analyses, wrote the paper and was involved in all measurements.
Supporting information
de Graaf TA, Thomson A, Janssens SEW, van Bree S, ten Oever S , Sack AT. Does alpha phase modulate visual target detection? Three experiments with tACS‐phase‐based stimulus presentation. Eur J Neurosci. 2020;51:2299–2313. 10.1111/ejn.14677
Edited by Prof. John Foxe.
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.14677
Funding information
This research was supported by the Netherlands Organization for Scientific Research (VICI grant 453‐15‐008 to AS, T.d.G.; VENI grant 451‐13‐024, S.J.; and Research Talent grant 406‐17‐540).
DATA AVAILABILITY STATEMENT
Data and code are available through the following link: https://hdl.handle.net/10411/CKP2SV.
REFERENCES
- Berger, H. (1929). Über das Elektroenkephalogramm des Menschen. Archiv Für Psychiatrie Und Nervenkrankheiten, 87, 527–570. [Google Scholar]
- Bishop, G. (1933). Cyclic changes in excitability of the optic pathway of the rabbit. American Journal of Physics, 103, 211–224. [Google Scholar]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Busch, N. A. , Dubois, J. , & VanRullen, R. (2009). The Phase of ongoing EEG oscillations predicts visual perception. Journal of Neuroscience, 29, 7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway, E. , & Yeager, C. L. (1960). Relationship between reaction time and electroencephalographic alpha phase. Science, 132, 1765–1766. 10.1126/science.132.3441.1765 [DOI] [PubMed] [Google Scholar]
- de Graaf, T. A. , Duecker, F. , Fernholz, M. H. P. , & Sack, A. T. (2015). Spatially specific vs. unspecific disruption of visual orientation perception using chronometric pre‐stimulus TMS. Frontiers in Behavioural Neurosciences, 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, T. A. , Duecker, F. , Stankevich, Y. , ten Oever, S. , & Sack, A. T. (2017). Seeing in the dark: Phosphene thresholds with eyes open versus closed in the absence of visual inputs. Brain Stimulation, 10, 828–835. 10.1016/j.brs.2017.04.127 [DOI] [PubMed] [Google Scholar]
- de Graaf, T. A. , Gross, J. , Paterson, G. , Rusch, T. , Sack, A. T. , & Thut, G. (2013). Alpha‐band rhythms in visual task performance: Phase‐locking by rhythmic sensory stimulation. PLoS ONE, 8, e60035 10.1371/journal.pone.0060035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, T. A. , Koivisto, M. , Jacobs, C. , & Sack, A. T. (2014). The chronometry of visual perception: Review of occipital TMS masking studies. Neuroscience and Biobehavioral Reviews, 45, 295–304. [DOI] [PubMed] [Google Scholar]
- de Graaf, T. A. , & Sack, A. T. (2011). Null results in TMS: From absence of evidence to evidence of absence. Neuroscience and Biobehavioral Reviews, 35, 871–877. [DOI] [PubMed] [Google Scholar]
- de Graaf, T. A. , & Sack, A. T. (2018). When and how to interpret null results in NIBS: A taxonomy based on prior expectations and experimental design. Frontiers in Neuroscience, 12, 458 10.3389/fnins.2018.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, J. , & VanRullen, R. (2011). This is the rhythm of your eyes: The phase of ongoing electroencephalogram oscillations modulates saccadic reaction time. Journal of Neuroscience, 31, 4698–4708. 10.1523/JNEUROSCI.4795-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué, L. , Marque, P. , & VanRullen, R. (2011). The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. Journal of Neuroscience, 31, 11889–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman, R. E. , & Beck, E. C. (1965). Phase of alpha brain waves, reaction time and visually evoked potentials. Electroencephalography and Clinical Neurophysiology, 18, 433–440. 10.1016/0013-4694(65)90123-9 [DOI] [PubMed] [Google Scholar]
- Fiebelkorn, I. C. , Foxe, J. J. , Butler, J. S. , Mercier, M. R. , Snyder, A. C. , & Molholm, S. (2011). Ready, set, reset: Stimulus‐locked periodicity in behavioral performance demonstrates the consequences of cross‐sensory phase reset. Journal of Neuroscience, 31, 9971–9981. 10.1523/JNEUROSCI.1338-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn, I. C. , Pinsk, M. A. , & Kastner, S. (2018). A dynamic interplay within the frontoparietal network underlies rhythmic spatial attention. Neuron, 99, 842–853.e8. 10.1016/j.neuron.2018.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn, I. C. , Pinsk, M. A. , & Kastner, S. (2019). The mediodorsal pulvinar coordinates the macaque fronto‐parietal network during rhythmic spatial attention. Nature Communications, 10, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallotto, S. , Sack, A. T. , Schuhmann, T. , & de Graaf, T. A. (2017). Oscillatory correlates of visual consciousness. Frontiers in Psychology, 8, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich, R. F. , Schneider, T. R. , Rach, S. , Trautmann‐Lengsfeld, S. A. , Engel, A. K. , & Herrmann, C. S. (2014). Entrainment of brain oscillations by transcranial alternating current stimulation. Current Biology, 24, 333–339. [DOI] [PubMed] [Google Scholar]
- Herrmann, C. S. , Strüber, D. , Helfrich, R. F. , & Engel, A. K. (2016). EEG oscillations: From correlation to causality. International Journal of Psychophysiology, 103, 12–21. [DOI] [PubMed] [Google Scholar]
- Iemi, L. , Chaumon, M. , Crouzet, S. M. , & Busch, N. A. (2017). Spontaneous neural oscillations bias perception by modulating baseline excitability. Journal of Neuroscience, 37, 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, C. , de Graaf, T. A. , & Sack, A. T. (2014). Two distinct neural mechanisms in early visual cortex determine subsequent visual processing. Cortex, 59, 1–11. 10.1016/j.cortex.2014.06.017 [DOI] [PubMed] [Google Scholar]
- Jaegle, A. , & Ro, T. (2014). Direct control of visual perception with phase‐specific modulation of posterior parietal cortex. Journal of Cognitive Neuroscience, 26, 422–432. [DOI] [PubMed] [Google Scholar]
- Kasten, F. H. , Dowsett, J. , & Herrmann, C. S. (2016). Sustained aftereffect of α‐tACS lasts up to 70 min after stimulation. Frontiers in Human Neuroscience, 10, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel, C. , Benwell, C. S. Y. , Thut, G. , & Gross, J. (2018). No changes in parieto‐occipital alpha during neural phase locking to visual quasi‐periodic theta‐, alpha‐, and beta‐band stimulation. European Journal of Neuroscience, 48, 2551–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S. P. , Lalor, E. C. , Reilly, R. B. , & Foxe, J. J. (2006). Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. Journal of Neurophysiology, 95, 3844–3851. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. , Sauseng, P. , & Hanslmayr, S. (2007). EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Research Reviews, 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Lange, J. , Oostenveld, R. , & Fries, P. (2013). Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. Journal of Neuroscience, 33, 3212–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. B. (1952). Psychological phenomena and the electroencephalogram. Electroencephalography and Clinical Neurophysiology, 4, 443–456. [DOI] [PubMed] [Google Scholar]
- Mathewson, K. E. , Fabiani, M. , Gratton, G. , Beck, D. M. , & Lleras, A. (2010). Rescuing stimuli from invisibility: Inducing a momentary release from visual masking with pre‐target entrainment. Cognition, 115, 186–191. 10.1016/j.cognition.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Mathewson, K. E. , Gratton, G. , Fabiani, M. , Beck, D. M. , & Ro, T. (2009). To see or not to see: Prestimulus α phase predicts visual awareness. Journal of Neuroscience, 29, 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson, K. E. , Lleras, A. , Beck, D. M. , Fabiani, M. , Ro, T. , & Gratton, G. (2011). Pulsed out of awareness: EEG alpha oscillations represent a pulsed‐inhibition of ongoing cortical processing. Frontiers in Psychology, 2, 99 10.3389/fpsyg.2011.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson, K. E. , Prudhomme, C. , Fabiani, M. , Beck, D. M. , Lleras, A. , & Gratton, G. (2012). Making waves in the stream of consciousness: Entraining oscillations in EEG alpha and fluctuations in visual awareness with rhythmic visual stimulation. Journal of Cognitive Neuroscience, 24, 2321–2333. 10.1162/jocn_a_00288 [DOI] [PubMed] [Google Scholar]
- Neuling, T. , Rach, S. , & Herrmann, C. S. (2013). Orchestrating neuronal networks: Sustained after‐effects of transcranial alternating current stimulation depend upon brain states. Frontiers in Human Neuroscience, 7, 161 10.3389/fnhum.2013.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience, 2011, 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei, V. , Brodbeck, V. , Michel, C. , Amedi, A. , Pascual‐Leone, A. , & Thut, G. (2008). Spontaneous fluctuations in posterior α‐Band EEG activity reflect variability in excitability of human visual areas. Cerebral Cortex, 18, 2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei, V. , Gross, J. , & Thut, G. (2010). On the role of prestimulus alpha rhythms over occipito‐parietal areas in visual input regulation: Correlation or causation? Journal of Neuroscience, 30, 8692–8697. 10.1523/JNEUROSCI.0160-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei, V. , Rihs, T. , Brodbeck, V. , & Thut, G. (2008). Resting electroencephalogram alpha‐power over posterior sites indexes baseline visual cortex excitability. NeuroReport, 19, 203–208. 10.1097/WNR.0b013e3282f454c4 [DOI] [PubMed] [Google Scholar]
- Ronconi, L. , Busch, N. A. , & Melcher, D. (2018). Alpha‐band sensory entrainment alters the duration of temporal windows in visual perception. Scientific Reports, 8, 11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi, L. , & Marotti, R. B. (2017). Awareness in the crowd: Beta power and alpha phase of prestimulus oscillations predict object discrimination in visual crowding. Consciousness and Cognition, 54, 36–46. 10.1016/j.concog.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Sauseng, P. , Klimesch, W. , Stadler, W. , Schabus, M. , Doppelmayr, M. , Hanslmayr, S. , … Birbaumer, N. (2005). A shift of visual spatial attention is selectively associated with human EEG alpha activity. European Journal of Neuroscience, 22, 2917–2926. [DOI] [PubMed] [Google Scholar]
- Schilberg, L. , Engelen, T. , ten Oever, S. , Schuhmann, T. , de Gelder, B. , de Graaf, T. A. , & Sack, A. T. (2018). Phase of beta‐frequency tACS over primary motor cortex modulates corticospinal excitability. Cortex, 103, 142–152. 10.1016/j.cortex.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Sherman, M. T. , Kanai, R. , Seth, A. K. , & VanRullen, R. (2016). Rhythmic influence of top‐down perceptual priors in the phase of prestimulus occipital alpha oscillations. Journal of Cognitive Neuroscience, 28, 1318–1330. [DOI] [PubMed] [Google Scholar]
- ten Oever, S. , de Graaf, T. A. , Bonnemayer, C. , Ronner, J. , Sack, A. T. , & Riecke, L. (2016). Stimulus presentation at specific neuronal oscillatory phases experimentally controlled with tACS: Implementation and applications. Frontiers in Cellular Neuroscience, 10, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Oever, S. , & Sack, A. T. (2015). Oscillatory phase shapes syllable perception. Proceedings of the National Academy of Sciences, 112, 15833–15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher, A. , Antunes, A. , & Saturnino, G. B. (2015). Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS. 2015 37th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., 2015, 222–225. [DOI] [PubMed] [Google Scholar]
- Thut, G. , Nietzel, A. , Brandt, S. A. , & Pascual‐Leone, A. (2006). α‐Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Cognitive Neuroscience, 26, 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Schyns, P. G. , & Gross, J. (2011). Entrainment of perceptually relevant brain oscillations by non‐invasive rhythmic stimulation of the human brain. Frontiers in Psychology, 2, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Veniero, D. , Romei, V. , Miniussi, C. , Schyns, P. , & Gross, J. (2011). Rhythmic TMS causes local entrainment of natural oscillatory signatures. Current Biology, 21, 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Van Dijk, H. , Schoffelen, J.‐M. , Oostenveld, R. , & Jensen, O. (2008). Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. Journal of Neuroscience, 28, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen, A. , Gross, J. , & Thut, G. (2015). Alpha power increase after transcranial alternating current stimulation at alpha frequency (α‐tACS) reflects plastic changes rather than entrainment. Brain Stimulation, 8, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, A. B. , & Pelli, D. G. (1983). QUEST: A Bayesian adaptive psychometric method. Perception & Psychophysics, 33(2), 113–120. [DOI] [PubMed] [Google Scholar]
- Worden, M. S. , Foxe, J. J. , Wang, N. , & Simpson, G. V. (2000). Anticipatory biasing of visuospatial attention indexed by retinotopically specific α‐bank electroencephalography increases over occipital Cortex. The Journal of Neuroscience, 20, RC63–RC63 10.1523/JNEUROSCI.20-06-j0002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available through the following link: https://hdl.handle.net/10411/CKP2SV.


