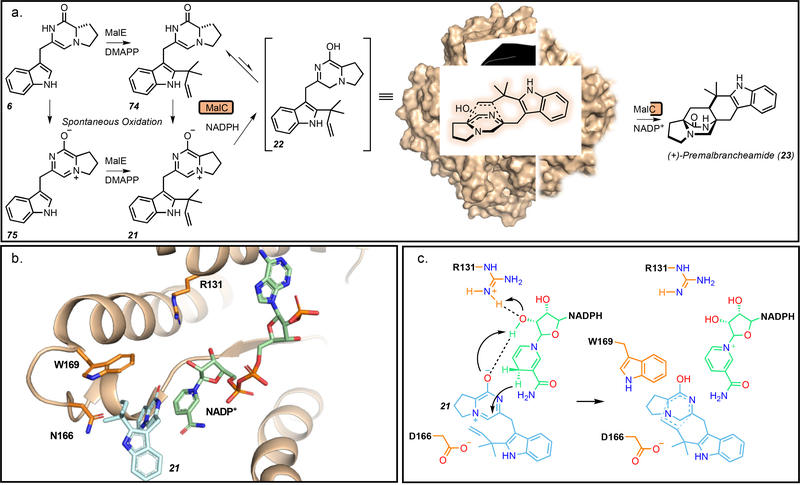
Figure 8.
a. Reactions performed by MalC. Reduction and stereochemical control are performed by MKP IMDAase MalC which “holds” the substrate in the proper conformation to generate (+)-premalbrancheamide (23). b. Active site of PhqE in complex with the prenyl zwitterion 21. c. Proposed mechanism involving donation of a proton from R131 and stabilization of the zwitterion by D166 followed by hydride transfer from the nicotinamide adenine dinucleotide phosphate (NADPH) cofactor to generate the unstable azadiene. The cofactor and W169 provide diastereo- and enantioselective control of the cycloaddition. Model generated using PyMol Molecular Graphics System.