This phase 1 and phase 2 randomized clinical trial seeks to determine the maximum tolerated doses of trastuzumab emtansine plus capecitabine in patients with previously treated ERBB2 (HER2)-positive metastatic breast cancer and locally advanced/metastatic gastric cancer (phase 1) and the efficacy and safety of this combination vs trastuzumab emtansine alone in patients with metastatic breast cancer (phase 2).
Key Points
Question
What is the effect of adding capecitabine to trastuzumab emtansine (T-DM1) treatment in patients with previously treated ERBB2 (HER2)-positive metastatic breast cancer?
Findings
In this phase 1/2 randomized clinical trial of 161 patients with previously treated ERBB2-positive metastatic breast cancer, the overall response rate was 44% and 36% in the combination and single-agent T-DM1 arms, respectively; median overall survival was not estimable and 24.7 months. Adverse events occurred in 95% (grade 3-4: 44%) and 89% (grade 3-4: 41%) of patients in each arm, respectively.
Meaning
Adding capecitabine to T-DM1 increases toxic effects and does not improve clinical outcomes vs T-DM1 alone for previously treated ERBB2-positive metastatic breast cancer.
Abstract
Importance
ERBB2 (HER2)-targeted therapy provides benefits in metastatic breast cancer (mBC) and gastric cancer, but additional treatments are needed to maximize efficacy and quality of life.
Objective
To determine maximum tolerated doses (MTDs) of trastuzumab emtansine (T-DM1) plus capecitabine in patients with previously treated ERBB2-positive mBC and locally advanced/metastatic gastric cancer (LA/mGC) (phase 1) and the efficacy and safety of this combination vs T-DM1 alone in patients with mBC (phase 2).
Design, Setting, and Participants
The MTD in phase 1 was assessed using a 3 + 3 design with capecitabine dose modification. Phase 2 was an open-label, randomized, international multicenter study of patients with mBC treated with T-DM1 plus capecitabine or T-DM1 alone. Eligible patients had previously treated ERBB2-positive mBC or LA/mGC with no prior chemotherapy treatment for advanced disease.
Interventions
Patients in the phase 1 mBC cohort received capecitabine (750 mg/m2, 700 mg/m2, or 650 mg/m2 twice daily, days 1-14 of a 3-week cycle) plus T-DM1 3.6 mg/kg every 3 weeks. Patients with LA/mGC received capecitabine at the mBC phase 1 MTD, de-escalating as needed, plus T-DM1 2.4 mg/kg weekly. In phase 2, patients with mBC were randomized (1:1) to receive capecitabine (at the phase 1 MTD) plus T-DM1 or T-DM1 alone.
Main Outcomes and Measures
The phase 1 primary objective was to identify the MTD of capecitabine plus T-DM1. The phase 2 primary outcome was investigator-assessed overall response rate (ORR).
Results
In phase 1, the median (range) age was 54.0 (37-71) and 57.5 (53-70) years for patients with mBC and patients with LA/mGC, respectively. The capecitabine MTD was identified as 700 mg/m2 in 11 patients with mBC and 6 patients with LA/mGC evaluable for dose-limiting toxic effects. In phase 2, between October 2014 and April 2016, patients with mBC (median [range] age, 52.0 [28-80] years) were randomized to receive combination therapy (n = 81) or T-DM1 (n = 80). The ORR was 44% (36 of 81 patients) and 36% (29 of 80 patients) in the combination and T-DM1 groups, respectively (difference, 8.2%; 90% CI, −4.5 to 20.9; P = .34; clinical cutoff, May 31, 2017). Adverse events (AEs) were reported in 78 of 82 patients (95%) in the combination group, with 36 (44%) experiencing grade 3-4 AEs, and 69 of 78 patients (88%) in the T-DM1 group, with 32 (41%) experiencing grade 3-4 AEs. No grade 5 AEs were reported.
Conclusions and Relevance
Adding capecitabine to T-DM1 did not statistically increase ORR associated with T-DM1 in patients with previously treated ERBB2-positive mBC. The combination group reported more AEs, but with no unexpected toxic effects.
Trial Registration
ClinicalTrials.gov Identifier: NCT01702558
Introduction
Overexpression of ERBB2 (formerly HER2) is associated with poor clinical outcomes in patients with solid tumors.1,2 Therapies targeted at ERBB2, such as trastuzumab, have significantly improved outcomes for patients with ERBB2-positive metastatic breast cancer (mBC) and metastatic gastric cancer (mGC).3,4,5,6,7,8,9 However, prognoses for patients with mBC or mGC remain poor, and additional treatments are needed.
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate combining trastuzumab and the cytotoxic microtubule-inhibitor DM1.10 In the phase 3 EMILIA study of patients with previously treated ERBB2-positive advanced breast cancer, T-DM1 conferred significantly longer progression-free survival (PFS) and overall survival (OS) vs lapatinib plus capecitabine,8 leading to the US approval of single-agent T-DM1 in this setting. T-DM1 was also investigated in patients with previously treated ERBB2-positive locally advanced (LA)/mGC or gastroesophageal junction cancer in the phase 2/3 GATSBY study, which found that T-DM1 alone was not superior to a taxane.11
Combination trastuzumab plus capecitabine has shown clinical activity in patients with previously treated ERBB2-positive mBC.12,13,14 Trastuzumab plus chemotherapy, including capecitabine, is the first-line standard-of-care treatment for ERBB2-positive advanced gastric cancer.6 In the phase 3 ToGA trial of patients with ERBB2-positive advanced gastric cancer, first-line trastuzumab plus chemotherapy (capecitabine plus cisplatin or fluorouracil plus cisplatin) improved OS and PFS vs chemotherapy alone, without substantially affecting safety.15
These studies, along with preclinical evidence showing additive activity when combining T-DM1 with chemotherapeutic agents,16 suggest that capecitabine plus T-DM1 may demonstrate efficacy in the treatment of mBC and LA/mGC. We conducted the TRAXHER2 trial (NCT01702558) to determine the maximum tolerated doses (MTDs) of T-DM1 plus capecitabine in ERBB2-positive mBC and LA/mGC and to explore efficacy of this combination vs T-DM1 monotherapy in patients with previously treated ERBB2-positive mBC.
Methods
Study Design and Patients
TRAXHER2 was a phase 1/2 study (trial protocol in Supplement 1; eFigure 1 in Supplement 2). Phase 1 assessed MTDs of capecitabine in combination with T-DM1 in patients with ERBB2-positive mBC (cohort 1) or LA/mGC (cohort 2) using a 3 + 3 classical study design with dose modification for capecitabine. Phase 2 was an international, open-label, randomized study of efficacy and safety of this combination in patients with ERBB2-positive mBC.
All patients were 18 years or older and had ERBB2-positive tumors, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower, adequate liver/renal function, left ventricular ejection fraction of 50% or greater, and life expectancy of 12 weeks or longer. Patients with mBC had at least 1 prior treatment for early or metastatic disease (including trastuzumab and chemotherapy, separately or in combination); those with LA/mGC had no prior chemotherapy for advanced disease. Additional inclusion and exclusion criteria are detailed in the eMethods in Supplement 2.
The TRAXHER2 study was conducted per the International Conference on Harmonization Good Clinical Practice standards and the Declaration of Helsinki. The study protocol was approved by the institutional review boards or ethics committees of participating institutions. All patients provided written informed consent. Patients were not paid for participating in the study; payments to patients for travel expenses and small refreshments were covered per local regulations.
Procedures
The phase 1 mBC cohort established the MTD, which was used as the starting capecitabine dose for the phase 1 LA/mGC cohort and as the recommended phase 2 dose (RP2D) for the combination arm. Although the phase 1 mBC cohort was initially designated as a dose-escalation study, because of dose-limiting toxic effects (DLTs) observed at dose level 1 in 2 patients, the study design was adapted for de-escalation. Three possible capecitabine dose levels were thus evaluated in the mBC cohort: 750 mg/m2 (dose level 1), 700 mg/m2 (dose level −1), and 650 mg/m2 (dose level −2) (eFigure 1 in Supplement 2). Capecitabine was given twice daily (days 1-14 of a 3-week cycle) plus T-DM1 3.6 mg/kg every 3 weeks.
Patients in the phase 1 LA/mGC cohort were also treated using a de-escalation design, starting with the capecitabine MTD from the phase 1 mBC cohort (700 mg/m2; dose level −1) (eFigure 1 in Supplement 2). Capecitabine was given twice daily (days 1-14 of a 3-week cycle) plus T-DM1 2.4 mg/kg weekly. Additional details are included in the eMethods in Supplement 2.
In phase 2, patients with mBC were randomized 1:1 to T-DM1 plus capecitabine (at phase 1 MTD) or T-DM1 alone (3.6 mg/kg every 3 weeks). Treatment continued until disease progression, unacceptable toxic effects, patient withdrawal, death, or at the discretion of the treating physician (eTable 1 in Supplement 2).
Tumors were assessed for response at 9 weeks and then every 12 weeks until progressive disease or study termination. Adverse events (AEs) were coded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. Additional procedural methods are included in the eMethods and eTable 1 in Supplement 2.
Outcomes
The phase 1 primary outcome was the MTD for T-DM1 plus capecitabine in mBC and LA/mGC. The secondary outcome was overall response rate (ORR) based on the best overall response per investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (unconfirmed).
The phase 2 primary outcome was investigator-assessed ORR using RECIST, version 1.1, with complete/partial responses (CR/PR) or stable disease (SD; ≥6-week duration from randomization) confirmed at a subsequent visit. Secondary end points were safety, PFS, OS, clinical benefit rate (confirmed CR/PR or SD≥6 months), time to response, duration of response, time to progression, and time to treatment failure. The ORR by ERBB2 subgroup was an exploratory end point.
Statistical Analysis
Phase 1 sample sizes were based on a classical 3 + 3 design with enrollment of 6 to 18 patients with mBC and 3 to 12 patients with LA/mGC. Phase 2 sample size was based on a Fisher exact test with an α level of 5% (1-sided) and power of 70%, and the clinical assumption of ORRs of 62.5% for T-DM1 plus capecitabine and 43% for T-DM1 alone. Based on these assumptions and a 15% withdrawal rate, approximately 160 patients (80 patients per group) were to be randomized.
The primary analysis of phase 2 was prespecified to occur when 70% of patients had experienced a PFS event. This clinical cutoff was applied on May 31, 2017, 31 months after first patient randomization, at which point the last patient randomized (April 26, 2016) could be adequately evaluated for best overall response. This allowed for calculation of the primary end point (ORR) and estimation of median PFS using the Kaplan-Meier method. This analysis represents final results from the TRAXHER2 study. Statistical analyses were performed using SAS, version 9 (SAS Institute).
Results
Patients
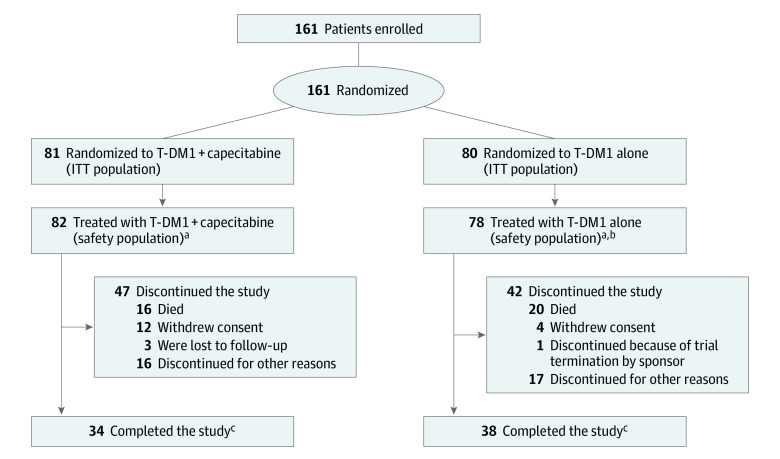
Baseline demographic and disease characteristics for all patients are summarized in eTable 2 in Supplement 2. In phase 1, 12 patients were enrolled in the mBC cohort, 11 of whom were evaluable for DLTs; the excluded patient did not meet eligibility criteria for liver enzyme stability. Six patients were enrolled in the LA/mGC cohort and evaluable for DLTs. In phase 2, between October 17, 2014, and April 29, 2016, 161 patients with ERBB2-positive mBC (median [range] age, 52.0 [28-80] years) were randomized to receive T-DM1 plus capecitabine (n = 81) or T-DM1 alone (n = 80) (Figure 1). In phase 2, patient and disease characteristics were well balanced between treatment arms and comparable to patients in phase 1. Most were white women with an ECOG performance status of 1 or lower, had visceral disease, and had 1 or fewer prior lines of mBC treatment. Of 6 patients in the phase 1 LA/mGC cohort, 3 (50%) had adenocarcinoma of the gastroesophageal junction. All 6 patients were white men with an ECOG performance status of 1 or lower.
Figure 1. Patient Disposition in Phase 2.
aOne patient randomized to the T-DM1 alone group also received capecitabine throughout the study and is included in the T-DM1 + capecitabine safety population.
bOne patient in the T-DM1 alone group was randomized in error and was not treated.
cPatients who completed the study were those patients in follow-up who had their last visit within the last 6 months prior to the clinical cutoff date (May 31, 2017).
ITT indicates intention-to-treat; T-DM1, trastuzumab emtansine.
Phase 1
Of 11 DLT-evaluable patients in the mBC cohort, 6 were treated at dose level 1 and 5 were treated at dose level −1. Two of 6 patients receiving dose level 1 experienced DLTs: one had grade 3 elevated alanine aminotransferase and aspartate aminotransferase levels, while the other had grade 3 vomiting and grade 1 nausea. None of the patients receiving dose level −1 had a DLT; therefore, the MTD and RP2D for mBC was identified as capecitabine 700 mg/m2 twice daily (days 1-14 of a 3-week cycle) plus T-DM1 3.6 mg/kg every 3 weeks. In the LA/mGC cohort, all 6 patients were treated at dose level −1; none experienced a DLT. Therefore, the MTD was capecitabine 700 mg/m2 twice daily (days 1-14 of a 3-week cycle) plus T-DM1 2.4 mg/kg weekly.
Median (interquartile range [IQR]) overall study drug exposure was 14.3 (7.7-25.3) months and 4.0 (2.6-7.4) months in the mBC and LA/mGC cohorts, respectively. Among 11 DLT-evaluable patients with mBC, 10 had unconfirmed PR (5 each at dose levels 1 and −1). In the LA/mGC cohort, 5 patients had an unconfirmed CR (n = 1) or PR (n = 4).
Phase 2
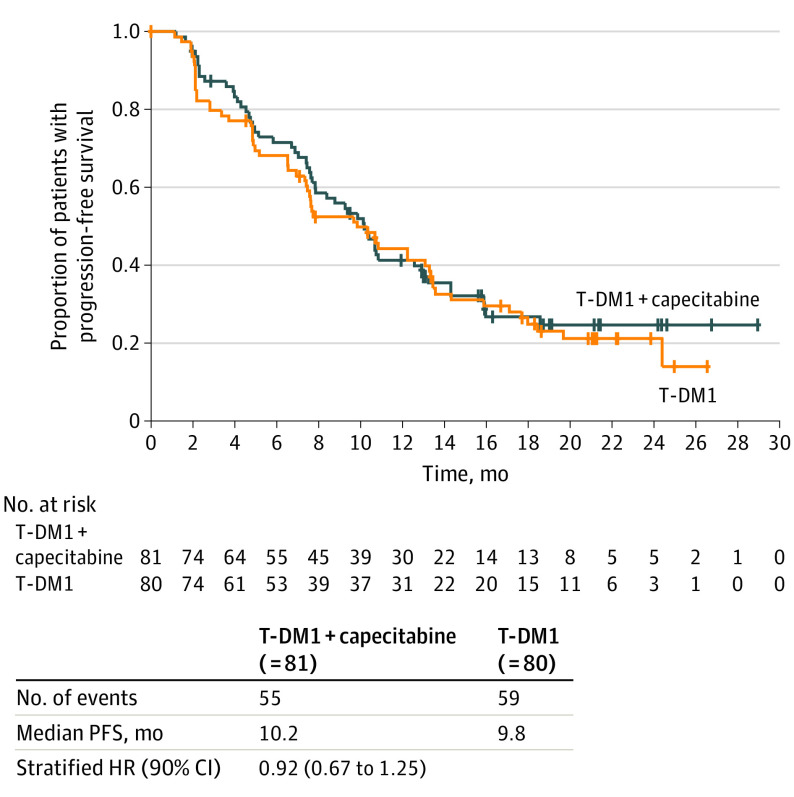
At the clinical cutoff, median (IQR) study drug exposure was 7.6 (4.1-13.8) months. The ORR was 44% (36 of 81) in the combination arm and 36% (29 of 80) in the T-DM1 arm (difference, 8.2%; 90% CI, −4.5 to 20.9; P = .34). Median PFS was 10.2 months (90% CI, 7.9-12.6) for the combination arm and 9.8 months (90% CI, 7.5-13.1) for the T-DM1 arm (stratified hazard ratio, 0.92; 90% CI, 0.67-1.25) (Figure 2). Median OS was not estimable (NE; 90% CI, NE-NE) for the combination arm and 24.7 months (90% CI, 24.3-NE) for the T-DM1 arm (stratified hazard ratio, 0.87; 90% CI, 0.51-1.48) (eFigure 2 in Supplement 2). Consistent results were seen for the other secondary efficacy end points (Table). In the exploratory analysis, ORR results were consistent within ERBB2 subgroups defined by immunohistochemistry level (eTable 3 in Supplement 2).
Figure 2. Progression-Free Survival in Patients With Metastatic Breast Cancer From Phase 2.
Patients without progression/death were censored at the date of the last tumor assessment. HR estimates and 90% CIs are from a Cox regression model stratified by the number of previous lines of treatment.
HR indicates hazard ratio; T-DM1, trastuzumab emtansine.
Table. Efficacy Results From Phase 2.
| T-DM1 + capecitabine (n = 81) | T-DM1 (n = 80) | |
|---|---|---|
| Overall response rate, No. (%) [90% CI] | 36 (44.4) [35.0 to 54.2] | 29 (36.3) [27.3 to 46.0] |
| Difference, % (90% CI) | 8.2 (−4.5 to 20.9) | |
| P value | .34 | |
| Clinical benefit rate, No. (%) [90% CI] | 54 (66.7) [57.1 to 75.3] | 50 (62.5) [52.7 to 71.6] |
| Best overall response, No. (%) | ||
| Complete response | 2 (2) | 2 (3) |
| Partial response | 34 (42) | 27 (34) |
| Stable disease | 24 (30) | 26 (33) |
| Progressive disease | 14 (17) | 23 (29) |
| Not evaluable | 7 (9) | 2 (3) |
| Time to response, median (IQR), moa | 2.10 (1.99 to 3.24) | 2.10 (2.04 to 4.67) |
| Duration of response, median (IQR), moa | 11.30 (8.18 to NE) | 12.22 (8.25 to 19.88) |
| Time to treatment failure, median (IQR), mo | 9.86 (4.67 to 15.87) | 7.66 (4.27 to 14.52) |
| Time to progression, median (IQR), mo | 10.38 (4.93 to NE) | 10.32 (4.83 to 18.43) |
Abbreviations: IQR, interquartile range; NE, not estimable; T-DM1, trastuzumab emtansine.
Provided for responders only.
In the combination and T-DM1 arms, 9 and 7 patients, respectively, had brain lesions at screening. In the combination arm, 2 patients had CR, 3 had PR, 2 had SD, and 2 had progressive disease. In the T-DM1 arm, 6 patients had SD in the brain and 1 had progressive disease. In the combination and T-DM1 arms, 1 and 6 patients, respectively, developed new brain lesions during the study.
The safety population included 82 patients in the combination arm and 78 in the T-DM1 arm (Figure 1). Two patients randomized to receive T-DM1 were not included in the safety population for this treatment arm: one received capecitabine and is included in the combination arm for the safety analysis; the other was randomized in error and was not treated.
Study termination was most commonly owing to death in both treatment arms (combination arm, 16 of 47 patients [34%]; T-DM1 arm, 20 of 42 patients [48%]; eTable 4 in Supplement 2). Treatment discontinuation was most commonly due to disease progression; in the combination arm (n = 81), 49 of 81 patients (61%) discontinued T-DM1 and 44 (54%) discontinued capecitabine with disease progression as the primary reason. In the T-DM1 arm (n = 80), disease progression was the primary reason for discontinuation of T-DM1 in 48 patients (60%). Other reasons for study/treatment discontinuation are presented in eTable 4 in Supplement 2.
Median (IQR) duration of T-DM1 exposure was 7.7 (3.5-13.4) months and 7.6 (4.2-14.5) months in the combination and T-DM1 arms, respectively. Median (IQR) duration of capecitabine exposure was 6.4 (3.7-12.8) months. Overall, 27 of 82 patients (33%) and 29 of 78 patients (37%) in the combination and T-DM1 arms, respectively, had a reduction in T-DM1 dose; 44 (54%) and 44 (56%) patients, respectively, had a T-DM1 dose delay. At last patient visit, 26 patients were still on treatment in phase 2 (14 in the combination arm; 12 in the T-DM1 arm), and 1 patient was still on combination treatment from phase 1 (mBC cohort; dose level −1).
Adverse events were reported by 78 of 82 (95%) and 69 of 78 (88%) patients in the combination and T-DM1 treatment arms, respectively, and grade 3-4 AEs were reported in 36 (44%) and 32 (41%) patients (eTable 5 in Supplement 2). No grade 5 AEs or unexpected safety issues were observed. The 3 most frequently occurring grade 3-4 AEs were thrombocytopenia (combination, 8 patients [10%]; vs T-DM1, 3 patients [4%]), increased AST (4 [5%] vs 5 [6%]), and increased γ-glutamyltransferase (4 [5%] vs 5 [6%]) (eTable 5 in Supplement 2). Three patients in each arm experienced grade 4 AEs, including thrombocytopenia, brain edema, and pulmonary embolism (combination arm), and thrombocytopenia, hepatocellular injury, and bacterial sepsis (T-DM1 arm). Study and/or treatment discontinuation owing to AEs was higher in the combination vs the T-DM1 arm (23 of 82 patients [28%] vs 12 of 78 patients [15%]). Adverse events leading to study/treatment discontinuation in 2% or greater of patients (regardless of treatment) were thrombocytopenia (combination, 5 [6%]; vs T-DM1, 3 [4%]), increased blood bilirubin (2 [2%] vs 2 [3%]), and decreased platelet count (2 [2%] vs 1 [1%]) (eTable 6 in Supplement 2).
Discussion
In phase 1, the MTD for mBC was capecitabine 700 mg/m2 twice daily (days 1-14 of a 3-week cycle) plus T-DM1 3.6 mg/kg every 3 weeks, which established the RP2D. For LA/mGC, the MTD was capecitabine 700 mg/m2 twice daily (days 1-14 of a 3-week cycle) plus T-DM1 2.4 mg/kg weekly. Unconfirmed responses were observed in both cohorts, including 1 CR (LA/mGC cohort). In phase 2, the addition of capecitabine to T-DM1 did not statistically increase the ORR vs T-DM1 alone in patients with previously treated ERBB2-positive mBC (ORRs, 44% and 36% in the combination and T-DM1 monotherapy groups, respectively). The T-DM1 safety profile was generally consistent with previous reports of T-DM1 use in this setting8,9,17,18,19; however, among AEs occurring in 15% or greater of either treatment arm, incidences of thrombocytopenia, palmar-plantar erythrodysesthesia, vomiting, nausea, epistaxis, and neutropenia were higher (≥5% difference) in the combination arm vs the T-DM1 arm. In addition, more patients in the combination arm discontinued treatment because of AEs. No grade 5 AEs or unexpected safety issues were observed in either treatment arm.
When TRAXHER2 was designed, we hypothesized that combining T-DM1 with capecitabine might improve efficacy in patients with previously treated mBC. Findings from the present study did not support this hypothesis. Two other trials have also investigated T-DM1 combined with chemotherapeutic agents in patients with ERBB2-positive advanced breast cancer.20,21 In study BP22572, T-DM1 plus docetaxel treatment resulted in an ORR of 80% (20 CRs/PRs of 25 treated patients).20 However, 12 patients (48%) required dose modifications owing to AEs, and higher severe/serious AE rates were observed relative to prior studies with single-agent T-DM1. In study TDM4652g, heavily pretreated patients received T-DM1 plus paclitaxel with or without pertuzumab; ORR was 50% (n = 21) in 42 patients (48% with T-DM1 plus paclitaxel and 52% with T-DM1 plus paclitaxel and pertuzumab).21 High rates of peripheral neuropathy led to the conclusion that it was unclear whether adding pertuzumab or paclitaxel enhanced clinical activity of single-agent T-DM1. These trials suggest either no or a modest improvement in efficacy when chemotherapy is added to T-DM1, with an increase in toxic effects. Similarly, we observed no improvement in efficacy from the addition of capecitabine to T-DM1 in TRAXHER2. Further studies may provide additional data, including a phase 1 study of T-DM1 plus nonpegylated liposomal doxorubicin (MEDOPP038; THELMA).22 Other studies are investigating targeted therapy combinations, including T-DM1 plus tyrosine kinase inhibitors.
Limitations
Our study was limited by several factors, including the small number of patients. In addition, as phase 1 responses were unconfirmed, these efficacy results should be interpreted with caution, especially when compared with confirmed response data from phase 2. Short follow-up duration also limits our ability to characterize potential long-lasting benefits in responding patients. Furthermore, while T-DM1 plus capecitabine could be hypothesized to provide enhanced benefit in the setting of ERBB2 expression heterogeneity, the level of ERBB2 heterogeneity in patients with mBC or LA/mGC included in TRAXHER2 is unknown. Finally, while 2 CRs and 3 PRs were observed among 9 patients with baseline brain lesions in the combination arm, it is not possible to draw conclusions about T-DM1 plus capecitabine efficacy in this population because of the small patient numbers.
Conclusions
The MTD for capecitabine, in combination with T-DM1, was identified as 700 mg/m2 in both the mBC and LA/mGC phase 1 cohorts. In phase 2, the addition of capecitabine to T-DM1 did not statistically increase the ORR associated with T-DM1 alone in previously treated ERBB2-positive mBC, although it is possible that different dose combinations or schedules may improve outcomes.
Trial Protocol
Supplementary Acknowledgments: TRAXHER2 Study Investigators
eMethods.
eFigure 1. TRAXHER2 Study Design
eFigure 2. Overall Survival in Patients With Metastatic Breast Cancer From Phase II
eTable 1. Protocol-Specified Administration of T-DM1 and Capecitabine and Rules for Dose Delays, Dose Reductions, and Dose Discontinuations in Phase II
eTable 2. Patient Demographics and Baseline Characteristics
eTable 3. Overall Response Rates by HER2 Subgroup From Phase II
eTable 4. Most Common Reasons for Study and Treatment Discontinuation
eTable 5. Safety Results From Phase II
eTable 6. AEs Leading to Discontinuation From Study or Study Treatment in ≥2 Patients in Either Treatment Arm
eReferences.
Data Sharing Statement
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182. doi: 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19(5):554-568. doi: 10.1081/CNV-100103852 [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. 2017;28(12):3111. doi: 10.1093/annonc/mdx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . Breast cancer. Version 2.2020. Accessed February 19, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 5.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-v49. doi: 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network . Gastric cancer. Version 4.2019. Accessed February 19, 2020. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- 7.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461-471. doi: 10.1016/S1470-2045(13)70130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S, Miles D, Gianni L, et al. ; EMILIA Study Group . Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783-1791. doi: 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743-754. doi: 10.1016/S1470-2045(17)30313-3 [DOI] [PubMed] [Google Scholar]
- 10.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280-9290. doi: 10.1158/0008-5472.CAN-08-1776 [DOI] [PubMed] [Google Scholar]
- 11.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640-653. doi: 10.1016/S1470-2045(17)30111-0 [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol. 2009;27(12):1999-2006. doi: 10.1200/JCO.2008.19.6618 [DOI] [PubMed] [Google Scholar]
- 13.Schaller G, Fuchs I, Gonsch T, et al. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007;25(22):3246-3250. doi: 10.1200/JCO.2006.09.6826 [DOI] [PubMed] [Google Scholar]
- 14.Ishida T, Kiba T, Takeda M, et al. Phase II study of capecitabine and trastuzumab combination chemotherapy in patients with HER2 overexpressing metastatic breast cancers resistant to both anthracyclines and taxanes. Cancer Chemother Pharmacol. 2009;64(2):361-369. doi: 10.1007/s00280-008-0882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 16.Lewis Phillips G, Fields CT, Crocker L, et al. Potent anti-tumor activity of trastuzumab-DM1 antibody conjugate in combination with cytotoxic chemotherapeutic agents, antibodies or small molecule kinase inhibitors [abstract]. Cancer Res. 2008;68(suppl):2133. [Google Scholar]
- 17.Diéras V, Harbeck N, Budd GT, et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. J Clin Oncol. 2014;32(25):2750-2757. doi: 10.1200/JCO.2013.54.4999 [DOI] [PubMed] [Google Scholar]
- 18.Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732-742. doi: 10.1016/S1470-2045(17)30312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montemurro F, Ellis P, Anton A, et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: primary results from the KAMILLA study cohort 1. Eur J Cancer. 2019;109:92-102. doi: 10.1016/j.ejca.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Fumoleau P, Dewar JA, et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann Oncol. 2016;27(7):1249-1256. doi: 10.1093/annonc/mdw157 [DOI] [PubMed] [Google Scholar]
- 21.Krop IE, Modi S, LoRusso PM, et al. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. 2016;18(1):34. doi: 10.1186/s13058-016-0691-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Miranda E, Brain E, Saura C, et al. Phase I multicenter clinical trial evaluating the combination of trastuzumab emtansine (T-DM1) and non-pegylated liposomal doxorubicin (NPLD) in HER2-positive metastatic breast cancer (MBC) (MEDOPP038 study) [abstract No. OT1-02-03]. Cancer Res. 2017;77(suppl 4). doi: 10.1158/1538-7445.SABCS16-OT1-02-03 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Supplementary Acknowledgments: TRAXHER2 Study Investigators
eMethods.
eFigure 1. TRAXHER2 Study Design
eFigure 2. Overall Survival in Patients With Metastatic Breast Cancer From Phase II
eTable 1. Protocol-Specified Administration of T-DM1 and Capecitabine and Rules for Dose Delays, Dose Reductions, and Dose Discontinuations in Phase II
eTable 2. Patient Demographics and Baseline Characteristics
eTable 3. Overall Response Rates by HER2 Subgroup From Phase II
eTable 4. Most Common Reasons for Study and Treatment Discontinuation
eTable 5. Safety Results From Phase II
eTable 6. AEs Leading to Discontinuation From Study or Study Treatment in ≥2 Patients in Either Treatment Arm
eReferences.
Data Sharing Statement