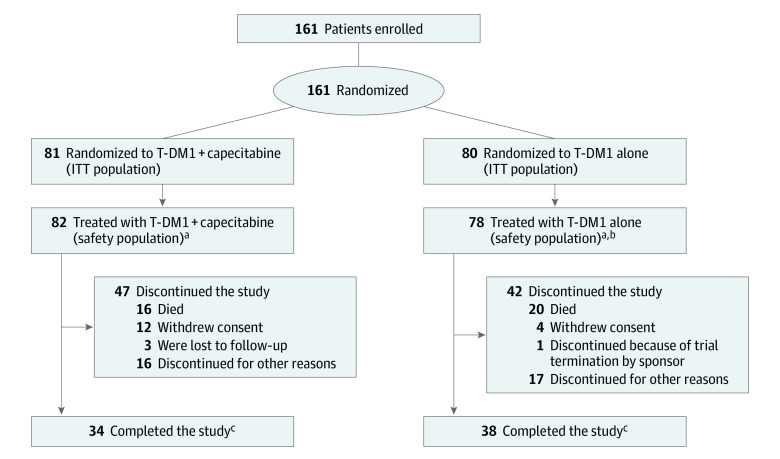
Figure 1. Patient Disposition in Phase 2.
aOne patient randomized to the T-DM1 alone group also received capecitabine throughout the study and is included in the T-DM1 + capecitabine safety population.
bOne patient in the T-DM1 alone group was randomized in error and was not treated.
cPatients who completed the study were those patients in follow-up who had their last visit within the last 6 months prior to the clinical cutoff date (May 31, 2017).
ITT indicates intention-to-treat; T-DM1, trastuzumab emtansine.