Figure 3.
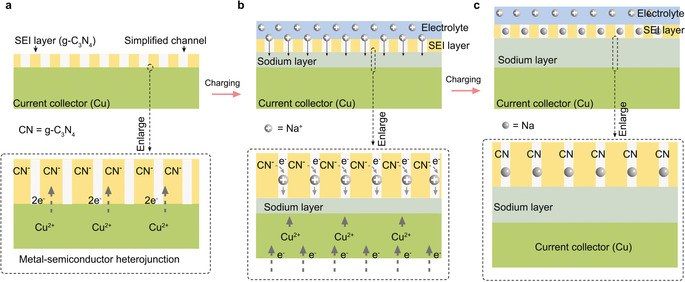
Illustration of the mechanism of the sodium storage in g‐C3N4 film activated Cu foil. a) The g‐C3N4 deposited on Cu foil (current collector) constitutes a metal–semiconductor heterojunction in which electrons will transfer from copper to g‐C3N4. b) In the charging process, Na+ ions permeate through thin g‐C3N4 films and deposit onto the Cu metal by underpotential deposition, thus forming there a Cu‐Na compound layer as sodium metal anode. c) The state after charging: sodium metal acts as the anode material and g‐C3N4 film works as SEI to avoid direct contact of the metallic sodium and liquid electrolyte.
