Abstract
Introduction
The diagnosis of inclusion body myositis (IBM) can be challenging, and its presentation can be confused with other forms of myositis or neuromuscular disorders. In this study we evaluate the ability of quantitative muscle ultrasound to differentiate between IBM and mimicking diseases.
Methods
Patients 50 years of age and older were included from two specialty centers. Muscle echogenicity and muscle thickness of four characteristically involved muscles in IBM were measured and compared with polymyositis (PM)/dermatomyositis (DM), other neuromuscular disorders, and healthy controls.
Results
Echogenicity was higher and muscle thickness generally lower in all four muscles in IBM compared with PM/DM and normal controls. When comparing IBM with the comparator groups, the flexor digitorum profundus was the most discriminative muscle.
Discussion
Ultrasound appears to be a good test to differentiate established IBM from PM/DM and neuromuscular controls, with value as a diagnostic tool for IBM.
Keywords: diagnosis, echogenicity, inclusion body myositis, neuromuscular disorders, quantitative muscle ultrasound
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- DM
dermatomyositis
- EI
echogenicity
- ENMC
European Neuromuscular Centre
- FDP
flexor digitorum profundus
- IBM
inclusion body myositis
- MT
muscle thickness
- PM
polymyositis
- QUMIA
Quantitative Muscle Imaging Analyzer
- ROC
receiver operating characteristic
- ROI
region of interest
- US
ultrasound
1. INTRODUCTION
The diagnosis of inclusion body myositis (IBM) can be challenging for clinicians, and its clinical presentation can be confused with other forms of myositis or other neuromuscular disorders.1, 2 Currently, the diagnosis of IBM is based on clinical, laboratory, and pathological features, but each of these may be inconclusive. Thus, there is still a need for additional diagnostic tools that are widely available, noninvasive, and easily deployed in a variety of settings. Quantitative muscle ultrasound (US) has the potential to differentiate between IBM and its mimicking diseases.3, 4, 5, 6, 7 Affected muscles show an increased echogenicity (EI) on the US image, reflecting replacement of muscle tissue with fat and fibrosis. 8 As IBM shows a specific pattern of muscle involvement, most commonly affecting the flexor digitorum profundus (FDP), gastrocnemius, and quadriceps muscles, 3 it may be possible to differentiate IBM from other diseases using this pattern.3, 4, 5, 6, 7
The aim of this study was to compare quantitative muscle US in IBM with other inflammatory myopathies and other clinically similar neuromuscular disorders.
2. METHODS
In this retrospective study we compared healthy controls, patients with myositis, and neuromuscular controls seen at the Neuromuscular Expertise Center, Radboudumc, Nijmegen, and the Johns Hopkins Myositis Center, Baltimore, Maryland. Patients at Radboudumc were imaged clinically for suspected disease and patients from Johns Hopkins were imaged for research and tended to have known disease. We included only subjects with definite diagnoses who were 50 years of age or older. All IBM patients met the European Neuromuscular Center (ENMC) criteria for clinicopathologically or clinically defined IBM. 9 All patients with dermatomyositis (DM) met the Bohan and Peter criteria for definite/probable DM,10, 11 ENMC criteria for definite/possible DM, 12 and/or carried an antibody specific for DM. All patients with polymyositis (PM) met the Bohan and Peter criteria for PM,10, 11 carried a myositis‐specific or ‐associated antibody, and were not otherwise classifiable as DM or IBM. For the neuromuscular controls, only patients who presented with proximal muscle weakness and/or finger flexion weakness, and in whom a diagnosis other than IBM was confirmed, were included. Body mass index (BMI) and duration of weakness were determined for all patients.
Muscle US was performed using a GE Logiq e ultrasound machine (GE, Fairfield, Connecticut) with a 12 L linear phased‐array transducer at Johns Hopkins, and an Esaote Mylab Twice machine (Esaote SpA, Genoa, Italy) with an LA533 linear 3‐ to 13‐MHz transducer at Radboudumc. We examined the four muscle groups with the best discriminative ability for IBM‐based previous studies3, 4, 5, 6, 7: the FDP, medial head of the gastrocnemius, rectus femoris, and/or vastus lateralis muscles. Muscles were examined bilaterally in all patients at Johns Hopkins and in most at Radboudumc. Bilateral examination results were averaged for statistical analysis. In cases where a bilateral examination was not performed, the unilateral results were used as representative of both sides.
System‐setting parameters were kept constant throughout the study, and scans were made using a strictly fixed preset and using protocols previously described for each center.3, 13 US images were analyzed using Quantitative Muscle Imaging Analysis (QUMIA, Radboudumc), a custom‐built software tool that allows for drawing regions of interest for gray‐scale histogram analysis and measuring muscle thickness with a digital caliper. Muscle thickness (MT) of the gastrocnemius, rectus femoris, and vastus lateralis was measured from the superior portion of the muscle to the lower muscle edge bounded by the fascia. For the FDP, thickness was measured from the midpoint of the muscle belly to the nearest perpendicular point of the ulna. All images from both centers were analyzed by the same examiner (K.L.), who was not study blind. Given the 2 different US systems and setups, individual EIs cannot be compared between centers, 13 but the use of an otherwise standardized protocol does allow for comparing the results at a group level. JMP software version 13 (SAS, Inc, Cary, North Carolina) was used for statistical analysis. Pooled t tests were used to determine differences between subgroups, and logistic regression using both US parameters of muscle EI and thickness with ROC analysis to evaluate the discriminative test quality per muscle group (area under the curve [AUC] 0.80 to 0.90 = good, AUC 0.90 to 1 = excellent).
Data from Radboudumc captured during routine clinical care were anonymized and needed no further ethical approval for post‐hoc review based on the general rules of the Dutch ethics committee. At Johns Hopkins, the study was approved by the institutional review board (IRB #00969260).
3. RESULTS
Baseline characteristics are shown in Table 1. In the Radboudumc cohort, subjects were slightly older in the IBM group compared with the other groups. In the Johns Hopkins cohort, there was a significantly longer duration of symptoms in patients with IBM than in those with PM/DM. When comparing the two cohorts, the duration of symptoms in IBM patients was significantly longer in the Johns Hopkins cohort compared with the Radboudumc cohort (P = .047).
TABLE 1.
Demographics of the Johns Hopkins and Radboudumc cohorts
| Disease group | Number of subjects | Male/female | Mean age, years (range) | BMI, kg/m2 (range) | Duration of symptoms, months (range) |
|---|---|---|---|---|---|
| Johns Hopkins | |||||
| IBM | 25 | 12/13 | 65.1 (52–80) | 25.5 (19.1–34.4) | 116.9 (30–360) |
| PM/DM | 21 | 5/16 | 67.0 (51–87) | 26.0 (17.3–39.5) | 66.6* (7–216) |
| Healthy controls | 25 | 9/16 | 65.9 (51–80) | 26.8 (19.7–36.8) | Not available |
| Radboudumc | |||||
| IBM | 16 | 9/7 | 70.5 (54–84) | 24.9 (19.6–33.6) | 67.2 (12–228) |
| PM/DM | 16 | 6/10 | 64.8 (54–76) | 26.9 (21.3–36.1) | 35.2 (1–276) |
| Neuromuscular controls | 11 | 6/5 | 65.2 (51–75) | 27.0 (22.6–41.4) | 50.2 (6–162) |
| Healthy controls | 63 | 28/35 | 63.4* (50–82) | 25.1 (18.8–34.9) | Not available |
BMI, body mass index; DM, dermatomyositis; IBM, inclusion body myositis; PM, polymyositis.
Neuromuscular controls: myopathies (n = 5), motor neuron diseases (n = 4), polyneuropathy (n = 1) and Lambert‐Eaton myasthenic syndrome (n = 1).
P < .05 vs IBM.
In the Radboudumc cohort, representing earlier disease, only the FDP showed a significantly higher EI in IBM compared with all controls (Figure 1). The vastus lateralis showed a difference between IBM patients, PM/DM patients, and healthy controls, but not when compared with the neuromuscular controls. The gastrocnemius and rectus femoris showed no difference in EI between IBM and other diseases. In the Johns Hopkins cohort, EI was significantly higher in the FDP, rectus femoris, and gastrocnemius in IBM patients compared with PM/DM patients and healthy controls. The vastus lateralis, which had smaller numbers, did not reach statistical significance for EI compared with PM/DM (Table 2).
FIGURE 1.
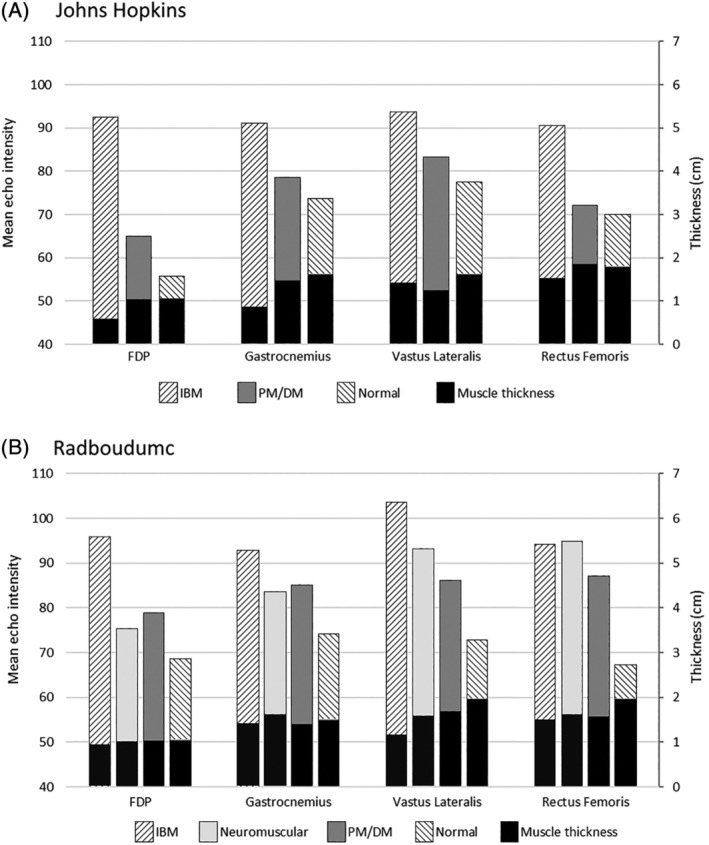
Mean muscle echogenicity (gray‐scale value 0–255) with muscle thickness (centimeters) across muscles for each disease group in the Johns Hopkins (A) and Radboudumc (B) cohorts. DM, dermatomyositis; FDP, flexor digitorum profundus; IBM, inclusion body myositis; PM, polymyositis
TABLE 2.
Echogenicity (gray‐scale value) and muscle thickness (centimeters) of each muscle per disease group
| Johns Hopkins | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disease group | Flexor digitorum profundus | Gastrocnemius | Rectus femoris | Vastus lateralis | ||||
| EI (SD) | MT (SD) | EI (SD) | MT (SD) | EI (SD) | MT (SD) | EI (SD) | MT (SD) | |
| IBM (N = 25) |
92.48 (14.75), N = 25 |
0.59 (0.24), N = 25 |
91.18 (12.43), N = 25 |
0.86 (0.49), N = 25 |
90.59 (14.24), N = 25 |
1.53 (0.47), N = 25 |
93.84 (14.99), N = 12 |
1.42 (0.51), N = 12 |
|
PM/DM (N = 21) |
65.07 † (10.85), N = 21 |
1.05 † (0.25), N = 21 |
78.45 † (13.12), N = 21 |
1.47 † (0.39), N = 21 |
72.11 † (11.56), N = 21 |
1.86* (0.39), N = 21 |
83.43 (6.02),N = 4 | 1.25 (0.17),N = 4 |
|
Healthy controls (N = 25) |
55,83 † (10.21) N = 25 |
1.06 † (0.23), N = 25 |
73.86 † (12.71), N = 25 |
1.61 † (0.28) N = 25 |
69.93* (13.57) N = 25 |
1.79* (0.42), N = 25 |
77.63* (10.41),N = 13 |
1.62 (0.29), N = 13 |
| Radboudumc | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disease group | Flexor digitorum profundus | Gastrocnemius | Rectus femoris | Vastus lateralis | ||||
| EI (SD) | MT (SD) | EI (SD) | MT (SD) | EI (SD) | MT (SD) | EI (SD) | MT (SD) | |
| IBM (N = 16) |
95.89 (13.33), N = 16 |
0.96 (0.31), N = 16 |
92.78 (17.97), N = 16 |
1.43 (0.32), N = 16 |
94.16 (6.95), N = 6 |
1.51 (0.36), N = 6 |
103.72 (17.98), N = 16 |
1.17 (0.10), N = 16 |
|
PM/DM (N = 16) |
78.86* (17.82), N = 16 |
1.04 (0.21), N = 16 |
85.15 (23.34), N = 16 |
1.41 (0.39), N = 16 |
87.17 (7.61), N = 5 |
1.57 (0.45), N = 5 |
86.13* (20.51), N = 16 |
1.70 † (0.10), N = 16 |
|
Neuromuscular controls (N = 11) |
75.41 † (12.58), N = 11 |
1.03 (0.34), N = 11 |
83.54 (16.89), N = 11 |
1.63 (0.44), N = 11 |
95.00 (16.68),N = 7 |
1.62 (0.27), N = 7 |
93.09 (11.60), N = 9 |
1.60* (0.35), N = 9 |
|
Healthy controls (N = 63) |
68.70 † (2.25), N = 28 |
1.04 (0.21), N = 28 |
74.25 † (8.23), N = 34 |
1.49 (0.07), N = 34 |
67.34 † (1.59), N = 61 |
1.96* (0.04), N = 61 |
72.91 † (12.28), N = 60 |
1.97 † (0.38), N = 60 |
DM, dermatomyositis; EI, echogenicity; IBM, inclusion body myositis; MT, muscle thickness; PM, polymyositis; SD, standard deviation.
P < .05 vs IBM.
P < .001 vs IBM.
In the Radboudumc cohort, only the vastus lateralis showed a significantly lower MT in IBM compared with the other disease groups. In the Johns Hopkins cohort, MT was significantly lower in all muscles (reflecting more atrophy) in IBM compared with PM/DM and healthy controls, except the vastus lateralis.
Logistic regression and ROC analysis with combined EI and MT showed that the vastus lateralis was the most discriminative for IBM when compared with PM/DM in the Radboudumc cohort (AUC 0.85), whereas the FDP was the most discriminative in the Johns Hopkins cohort (AUC 0.95). However, when compared with the neuromuscular controls, the FDP was more discriminative for IBM in the Radboudumc cohort (AUC = 0.86) than the vastus lateralis (AUC = 0.78).
4. DISCUSSION
In this study we have shown that quantitative muscle US of the deep finger flexors and quadriceps muscles provides good to excellent discrimination of patients with IBM from other inflammatory myopathies and clinically similar neuromuscular disorders.
Previous studies have examined different candidate muscle groups, but those studies were single‐center investigations with small patient numbers.3, 4, 5, 7 Based on our results, the most discriminating muscle for IBM compared with all the comparator groups was the FDP. This confirms other studies advocating the use of diagnostic muscle US for IBM using this muscle.6, 7 In addition, there is a relative sparing of the adjacent flexor carpi ulnaris that allows for comparison of these muscles in a single image, which would improve diagnostic accuracy for IBM during visual analysis. 4 However, because an increase in EI of the FDP (without accompanying atrophy) has been noted in muscular dystrophies, 14 the importance of considering US findings in light of the entire clinical assessment must be emphasized. As some patients present with predominant weakness of the lower extremities, the vastus lateralis would be the next best candidate. This may be particularly relevant in earlier disease, as noted in the Radboud cohort.
This work highlights the value of muscle US in the evaluation of patients with IBM. The difference in duration of disease can explain the slight differences seen in each cohort, but it also informs us about the progression of disease as seen on US. More significant differences in EI and MT are noted with longer disease duration. Given the frequency of initial misdiagnosis of IBM, US is still beneficial in confirming the diagnosis in later stages. In this study, care was taken to include only subjects aged 50 and over to ensure that differences seen were not accounted for by age; however, due to small numbers, sex differences were not explored in this study. The limitations of our study are its inclusion of patients with a known disease; the relatively longer disease duration of patients in the Hopkins cohort, thus limiting diagnostic utility; the variability in the number of muscles studied; and neuromuscular controls that came from only one center.
Overall, our study show that US is a good to excellent test for distinguishing established IBM from PM/DM and other neuromuscular disorders. A major next step would be a prospective study in a population of patients with early/suspected IBM.
CONFLICT OF INTEREST
N.v.A. is a muscle ultrasound consultant for Dynacure with a fee payment to the employer. The other authors declare no potential conflicts of interest.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Leeuwenberg KE, van Alfen N, Christopher‐Stine L, et al. Ultrasound can differentiate inclusion body myositis from disease mimics. Muscle Nerve. 2020;61:783–788. 10.1002/mus.26875
Christiaan G.J. Saris and Jemima Albayda contributed equally to this study as last authors.
Funding information Jerome L. Greene Foundation, Prinses Beatrix Muscle Foundation (to K.L.); Jerome L. Greene Foundation (to J.A., E.T., and C.M.); Johns Hopkins University (Clinician Scientist Award to C.M.); Rheumatology Research Foundation (to J.P.); Huayi and Siuling Zhang Discovery Fund (to L.C.S.).; Prinses Beatrix Spierfonds
Contributor Information
Christiaan G.J. Saris, Email: C.Saris@radboudumc.nl.
Jemima Albayda, Email: jalbayd1@jhmi.edu.
REFERENCES
- 1. Needham M, Corbett A, Day T, Christiansen F, Fabian V, Mastaglia FL. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci. 2008;15:1350‐1353. [DOI] [PubMed] [Google Scholar]
- 2. Engel WK, Askanas V. Inclusion‐body myositis: clinical, diagnostic, and pathologic aspects. Neurology. 2006;66(Suppl):S20‐S29. [DOI] [PubMed] [Google Scholar]
- 3. Albayda J, Christopher‐Stine L, Bingham CO, et al. Pattern of muscle involvement in inclusion body myositis: a sonographic study. Clin Exp Rheumatol. 2018;36:996‐1002. [PMC free article] [PubMed] [Google Scholar]
- 4. Noto Y‐I, Shiga K, Tsuji Y, et al. Contrasting echogenicity in flexor digitorum profundus‐flexor carpi ulnaris: a diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle Nerve. 2014;49:745‐748. [DOI] [PubMed] [Google Scholar]
- 5. Nodera H, Takamatsu N, Matsui N, et al. Intramuscular dissociation of echogenicity in the triceps surae characterizes sporadic inclusion body myositis. Eur J Neurol. 2016;23:588‐596. [DOI] [PubMed] [Google Scholar]
- 6. Vu Q, Cartwright M. Neuromuscular ultrasound in the evaluation of inclusion body myositis. BMJ Case Rep. 2016;2016 10.1136/bcr-2016-217440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karvelas KR, Xiao T, Langefeld CD, et al. Assessing the accuracy of neuromuscular ultrasound for inclusion body myositis. Muscle Nerve. 2019;59:478‐481. [DOI] [PubMed] [Google Scholar]
- 8. Pillen S, van Alfen N. Skeletal muscle ultrasound. Neurol Res. 2011;33:1016‐1024. [DOI] [PubMed] [Google Scholar]
- 9. Rose MR. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013;23:1044‐1055. [DOI] [PubMed] [Google Scholar]
- 10. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344‐347. [DOI] [PubMed] [Google Scholar]
- 11. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403‐407. [DOI] [PubMed] [Google Scholar]
- 12. Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC International Workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:337‐345. [DOI] [PubMed] [Google Scholar]
- 13. van Alfen N, Mah JK. Neuromuscular ultrasound: a new tool in your toolbox. Can J Neurol Sci. 2018;45:504‐515. [DOI] [PubMed] [Google Scholar]
- 14. Karvelas K, Hommel A, Cartwright M, Walker F, Hobson‐Webb L. Sonographic similarities of inclusion body myositis and myotonic dystrophy. Muscle Nerve. 2018;58:E25‐E26. [DOI] [PubMed] [Google Scholar]


