Scheme 2.
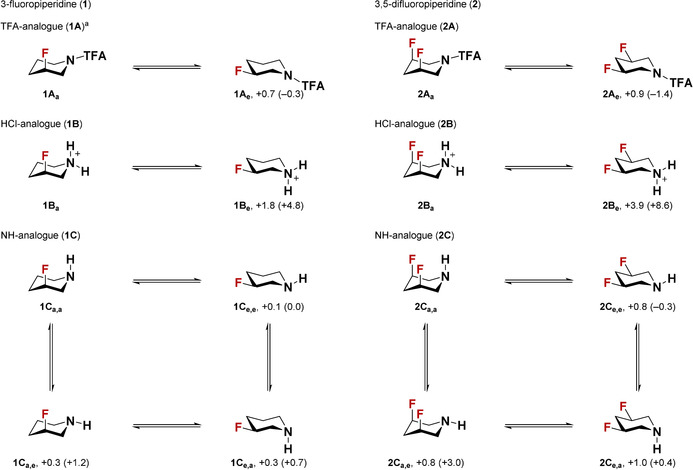
The conformational preferences of 3‐fluoropiperidine (1) and 3,5‐difluoropiperidine (2) and their TFA‐(A), HCl‐(B), and NH‐(C)‐analogues. The free enthalpy differences between the equatorial conformer to the axial conformer (ΔG) are presented as follows: ΔG Solvent (ΔG Gas Phase). The ΔG values for TFA‐, and for both HCl‐, and NH‐analogues are given in chloroform and water, respectively. All values are given in kcal mol−1. Experimentally, all analogues of 1 and 2 showed high axial preference. In NH‐analogues 1C and 2C, we were unable to determine the orientation of the N−H bond because of a fast H/D exchange in solution. [a] Both computational analysis and experimental observation were carried out in toluene.
