Figure 1.
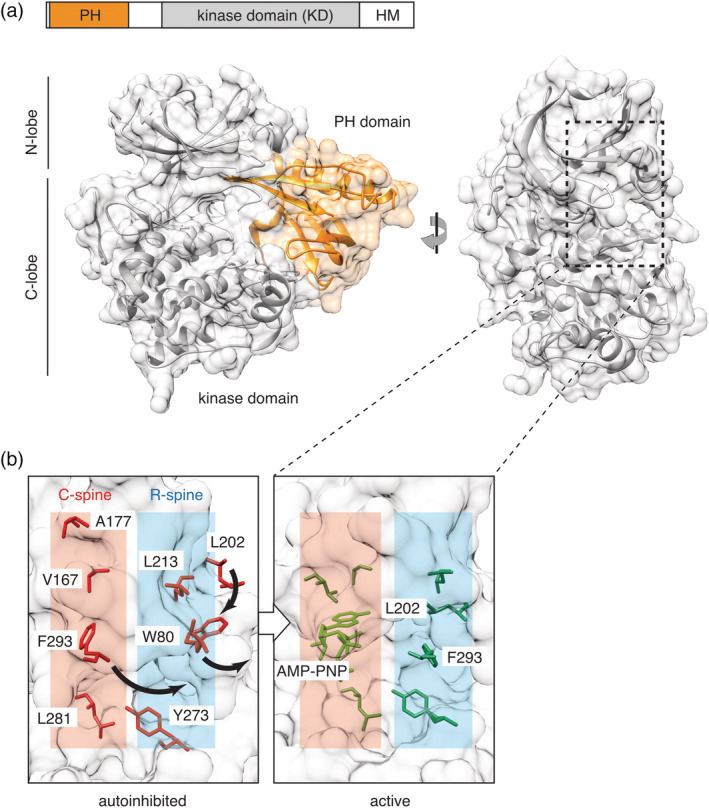
Akt structure and activation. (a) In the autoinhibited conformation, stabilized by the allosteric inhibitor VIII (PDB: 3o96), the lipid‐binding pleckstrin homology (PH) domain (orange) forms an extensive allosteric interface with the C‐lobe of the kinase domain (KD, gray). In the rotated view, the PH domain is hidden for clarity. Location of the C‐ and R‐spines connecting the N‐ and C‐lobes of the kinase domain is shown by dotted rectangle. (b) Akt phosphorylation on T308/S473 and allosteric mechanism cooperate to activate the kinase by aligning residues in the conserved hydrophobic spines. In the autoinhibited conformation (PDB: 3o96), W80 and F293 occupy the corresponding positions of L202 and nucleotide in the C‐ and R‐spines of the active form (PDB: 1o6k). Residues comprising the C‐ and R‐spines are highlighted; numbering corresponds to human Akt1 isozyme
