Figure 2.
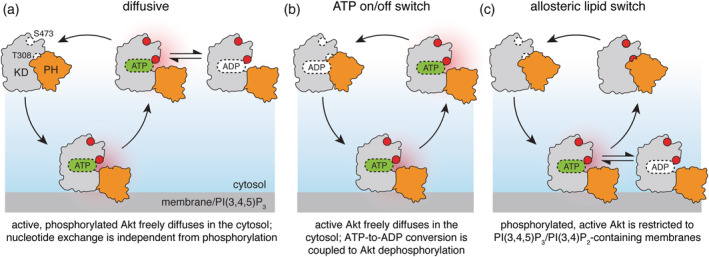
Models of intracellular Akt activation cycle. For all models, Akt activation requires binding to cellular membranes, containing PI(3,4,5)P3 and/or PI(3,4)P2 phosphoinositide lipids, accompanied by Akt phosphorylation on T308 and S473 (open and red‐filled circles) by membrane‐bound PDK1 and mTORC2 (not shown). PH domain is shown in orange, kinase domain in gray; red halo refers to catalytically active Akt. According to the diffusive model (a), phosphorylated, active Akt may dissociate from the membrane and diffuse in the cytosol phosphorylating the substrates (not shown) through multiple rounds of catalysis. An extension of the diffusive model, ATP on/off switch (b), links Akt dephosphorylation with the exchange of ATP for ADP during a single round of phosphate transfer onto the substrate. The allosteric lipid switch model (c) proposes that only membrane‐bound Akt is both phosphorylated and active, phosphorylating the substrates (not shown) in multiple rounds of catalysis. Dissociation from the membrane results in formation of the autoinhibited conformation and promotes rapid Akt dephosphorylation in the cytosol
