Abstract
The guidance issued to the pharmaceutical industry by the US Food and Drug Administration in 2008 has led to the publication of a series of randomized, controlled cardiovascular outcomes trials with newer therapeutic classes of glucose‐lowering medications. Several of these trials, which evaluated the newer therapeutic classes of sodium‐glucose co‐transporter‐2 inhibitors and glucagon‐like peptide‐1 receptor agonists, have reported a reduced incidence of major adverse cardiovascular and/or renal outcomes, usually relative to placebo and standard of care. Metformin was the first glucose‐lowering agent reported to improve cardiovascular outcomes in the UK Prospective Diabetes Study (UKPDS) and thus became the foundation of standard care. However, as this clinical trial reported more than 20 years ago, differences from current standards of trial design and evaluation complicate comparison of the cardiovascular profiles of older and newer agents. Our article revisits the evidence for cardiovascular protection with metformin and reviews its effects on the kidney.
Keywords: atherosclerotic cardiovascular disease, cardiovascular outcomes, chronic kidney disease, glucose‐lowering therapy, metformin
1. INTRODUCTION
Recent years have seen the publication of a series of large, randomized cardiovascular outcomes trials (CVOTs) of glucose‐lowering therapies.1 The transatlantic recommendations on diabetes management from the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) have, at the time of writing, retained metformin at the head of the algorithm for pharmacologic management of type 2 diabetes.2 However, the debate continues on the relative merits of metformin and the newer classes of sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors and glucagon‐like peptide‐1 (GLP‐1) receptor agonists for this position, especially for patients who already have manifestations of cardiovascular (CV) or renal disease.3
Metformin has been in clinical use for more than 60 years,4, 5, 6 and was the first glucose‐lowering agent associated with improved CV outcomes in a randomized trial; however, these data, from the UK Prospective Diabetes Study (UKPDS), were published more than 2 decades ago.7 Changes in trial design, and the greater availability of trial data for the newer glucose‐lowering agents, complicate comparisons of the relative therapeutic profiles of these agents and metformin. In this review, we seek to re‐evaluate the evidence for CV protection with metformin and discuss its place in future diabetes management algorithms, focusing mainly on the results of randomized controlled trials (RCTs).
2. METFORMIN IN ATHEROSCLEROTIC VASCULAR DISEASE
2.1. The UK Prospective Diabetes study
2.1.1. Randomization of newly diagnosed, overweight patients with type 2 diabetes to metformin (UKPDS34)
The UKPDS remains the principal source of trial evidence to support the presence of cardioprotective effects of metformin.7 Individuals eligible for randomization had newly diagnosed type 2 diabetes with fasting plasma glucose (FPG) >6.0 and <15 mmol/L after a 3‐month run‐in on a diet high in carbohydrates and low in saturated fats. Patients with active CV disease were excluded (recent myocardial infarction [<1 year]; angina or heart failure; >1 major vascular event). Figure 1 summarizes the randomization of patients in the study.7, 8 Randomization was stratified partially by weight: in 15 of the 23 UKPDS centres, 1709 overweight patients (>120% “ideal bodyweight”) were randomized to receive open‐label metformin (n = 342), “conventional” treatment (n = 411; this was essentially diet treatment and no placebo was used), or intensive glycaemic management with sulphonylurea (SU) or insulin (n = 961). The evaluation of metformin in the UKPDS is sometimes mistakenly referred to as a substudy; it is important to note that metformin was included within the primary trial randomization as part of the original protocol, although only in overweight patients, and only in the first 15 (of 23) centres.7 HbA1c was generally low (mean 7.2% [55 mmol/mol] at baseline) in this newly diagnosed population compared with most current trials in more longstanding type 2 diabetes, and the mean body mass index at baseline (31.4 kg/ms2) was consistent with a population that was obese, on average.
Figure 1.
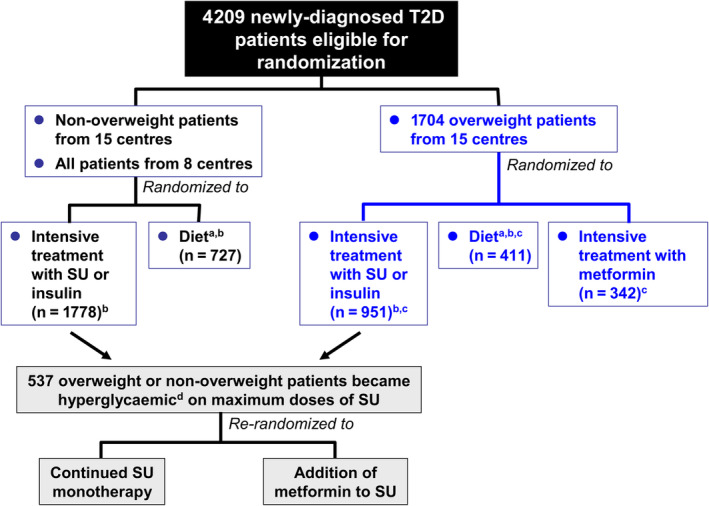
Summary of randomized allocation of patients to treatment in the UK Prospective diabetes study. SU, sulphonylurea; T2D, type 2 diabetes. aConventional treatment policy in the UKPDS. bThese patients were included in the main trial analysis (UKPDS 33). cUKPDS 34. dDefined as fasting plasma glucose 6.1–15 mmol/L (110–270 mg/dL) without symptoms of hyperglycaemia. Adapted from references7, 8 with permission from Elsevier
Participants were followed for a median of 10.7 years for clinical outcomes, which included aggregate outcomes, such as “any diabetes‐related endpoint” and “diabetes‐related death”, as well as individual outcomes (Figure 2). These two outcomes and all‐cause mortality were the three primary endpoints in the trial. Randomization to metformin was associated with reduced risk of any diabetes‐related endpoint (by 32%, P = 0.0023), all‐cause mortality (by 36%, P = 0.001) and diabetes‐related death (by 42%, P = 0.011) compared with the control group (Figure 2). Significant reductions were also reported for the risk of myocardial infarction (by 39%, P = 0.01) and all‐cause mortality (by 36%, P = 0.011). The risk of a combined macrovascular endpoint (myocardial infarction [MI], sudden death, angina, stroke and peripheral vascular disease) was reduced by 30% (5–48) in the metformin group, relative to control (P = 0.020); perhaps this is as close as we come to a modern major adverse cardiovascular event (MACE) outcome in the UKPDS.
Figure 2.
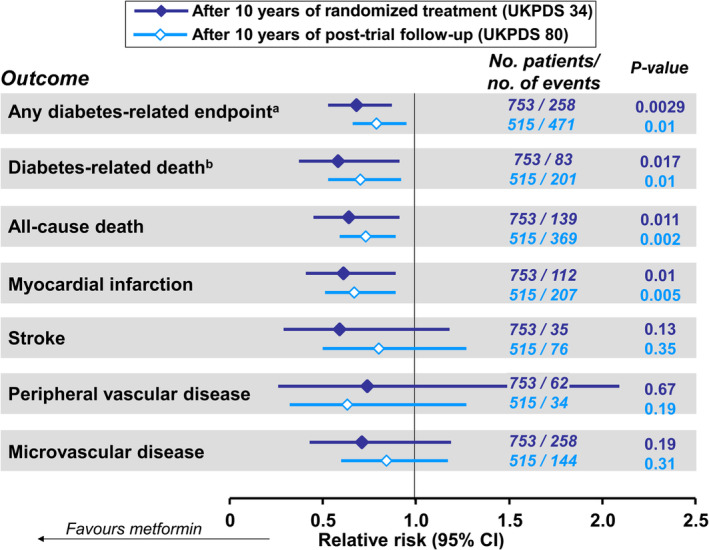
Relative risks of principal clinical outcomes from patients randomized to intensive glycaemic management with metformin or to conventional management policy in the UKPDS, and from patients previously in these randomized groups after 10 years of post‐trial follow‐up. aComposite of sudden death, death from hyperglycaemia or hypoglycaemia, fatal or non‐fatal myocardial infarction, angina, heart failure, stroke, renal failure, amputation (≥1 digit), vitreous haemorrhage, retinopathy requiring photocoagulation, blindness in one eye, or cataract extraction. bDeath from myocardial infarction, stroke, peripheral vascular disease, renal disease, hypoglycaemia or hyperglycaemia, and sudden death. Drawn from data presented in references7, 10
2.1.2. Rerandomization of patients hyperglycaemic on SU to additional metformin
Patients in the main metformin analysis of the UKPDS received additional SU if they became markedly hyperglycaemic (FPG >15 mmol/L or symptoms of hyperglycaemia), but remained within the metformin cohort for analysis. In addition, in a UKPDS substudy, 537 patients who became hyperglycaemic without symptoms on a maximal dose of SU, according to a lower cut‐off for glycaemia (FPG >6 mmol/L, equivalent to 108 mg/dL), were further randomized to addition of metformin, or to continue SU monotherapy (Figure 1).7, 8 The median follow‐up in this substudy was 6.6 years. Unexpectedly, there were significant increases for the combination versus the SU monotherapy group in all‐cause death (RR 1.60 [95% CI 1.02 to 2.52], P = 0.041) and diabetes‐related death (RR 1.96 [95% CI 1.02 to 3.75], P = 0.039). No other outcome was influenced significantly by addition of metformin versus continued SU monotherapy, including myocardial infarction (RR 1.09 [95% CI 0.67 to 1.78], P = 0.73).
These data were difficult to interpret and may have been affected by the play of chance. The UKPDS investigators later found that the mortality rate in the SU monotherapy group of the combination substudy (8.6/1000 patient years) was markedly lower than that seen in the main UKPDS population (11.0/1000 patient years).7, 9 By way of illustration, 35 deaths would have been predicted during 6.6 years of follow‐up on SU monotherapy, in contrast to only 26 deaths actually observed on the metformin‐SU combination (and only 14 deaths on SU monotherapy). A Cox proportional hazards model, with current therapies as a time‐dependent variable, found a numerical but not statistically significant effect of SU + metformin on diabetes‐related death, compared with all other treatments following adjustment for age, gender, ethnicity and FPG.7 Finally, in longer‐term follow‐up (see below) there was no adverse effect of metformin + SU on mortality outcomes, compared with SU monotherapy.10, 11
2.1.3. Long‐term post‐trial follow‐up
UKPDS participants were followed for outcomes for a further 10 years after the end of randomized treatment.10 The between‐group difference in HbA1c during the randomized phase diminished rapidly, with mean HbA1c similar during the rest of the follow‐up period in those previously randomized to intensive glycaemic management with metformin or to conventional treatment. The four outcomes for which significant risk reductions occurred in metformin‐treated patients in the randomized phase remained significantly reduced after post‐trial follow‐up (Figure 2). There was no significant effect on the incidence of stroke, peripheral vascular disease or microvascular disease (when considered as individual outcomes) in either phase.
2.2. Other studies in type 2 diabetes
While the UKPDS was conducted exclusively in newly diagnosed patients, another small, randomized trial (the HOME trial) enrolled a population with more advanced type 2 diabetes.12 Here, 390 participants with an average diabetes duration of 13 years, all on insulin treatment (for an average of ~ 6 years), were randomized to receive metformin or placebo (in addition to insulin) for an average follow‐up of 4.3 years. The primary endpoint was a composite of three microvascular and 13 macrovascular outcomes. Although the investigators attempted to maintain glycaemic equipoise between the metformin and placebo arms during blinded study treatment, there was a 0.4% (2 mmol/mol) reduction in HbA1c for metformin versus placebo.
There was no significant difference between groups for the primary microvascular and macrovascular composite endpoint (HR 0.92 [0.72 to 1.18], P = 0.33, after adjustment for baseline differences in age, gender, smoking and CV history) or the secondary composite microvascular endpoint (HR 1.04 [0.75 to 1.44], P = 0.43, after adjustment for age, gender, smoking and prior diabetic polyneuropathy). The secondary macrovascular composite endpoint was reduced significantly by metformin versus placebo (HR 0.61 [0.40 to 0.94], P = 0.02, adjusted as for the primary endpoint, above). In a mediation analysis, this was partly explained by a mean reduction of 3.07 kg bodyweight in the metformin group compared with placebo. The number needed to treat to prevent one macrovascular event was 16.
A 3‐year, randomized clinical trial conducted in China compared the effects of metformin and the SU, glipizide, on CV outcomes in 304 individuals with type 2 diabetes and pre‐existing coronary artery disease (documented prior MI or stenosis of ≥50% in a major coronary artery).13 Average diabetes duration was ~ 6 years, and 9% were receiving insulin. The primary outcome, an expanded MACE endpoint (CV death, all‐cause death, non‐fatal MI, non‐fatal stroke, or arterial revascularization) was reduced in the metformin group relative to glipizide (HR 0.54 [0.30 to 0.90], P = 0.026).
ADOPT (A Diabetes Outcome Progression Trial) randomized newly diagnosed people with type 2 diabetes to double‐blind treatment with metformin (n = 1454), rosiglitazone (n = 1456) or glibenclamide (glyburide, n = 1441) as initial antidiabetic pharmacotherapy, for 4 years.14 As this was not a prospective outcomes trial, it is included here for completeness: its primary outcome was a measure of glycaemic durability, with CV events (MI, stroke or heart failure) recorded as adverse events rather than being listed as prespecified outcomes. In this trial, 62 CV events occurred in the rosiglitazone group, 58 in the metformin group and 41 in the glibenclamide group.
Finally, the randomized Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) study (clinicaltrials.gov NCT01794143) is a parallel group study that is evaluating glimepiride, sitagliptin, liraglutide and insulin glargine, added to existing metformin therapy, over a follow‐up duration of 4–7 years in a population of 5047 people with a mean duration of type 2 diabetes of <5 years.15, 16 The primary outcome relates to durability of glycaemic control (HbA1c ≥7.0%/53 mmol/mol or ≥7.5%/59 mmol/mol); CV events and mortality are included as secondary outcome measures. As would be expected in this population, hypertension (67%) and hyperlipidaemia (72%) were prevalent at baseline. In contrast to the UKPDS, 69% and 66%, respectively, were on antihypertensive and lipid‐lowering treatment.17 However, given that GRADE was not powered as a CVOT, its results (expected in mid‐2021) will probably provide information on CV safety in contemporary metformin‐treated individuals with type 2 diabetes rather than definitive conclusions on the CV superiority or otherwise of any of the four “add‐on” strategies tested.
2.3. Studies in people with type 1 diabetes
Although metformin is indicated only for the management of type 2 diabetes, and direct extrapolation of effects to type 2 diabetes would be speculative, effects of the drug on the CV system in patients with type 1 diabetes are of interest in that they may help to shed light on the mechanisms of CV protection.
CV disease is receiving increasing attention as a cause of morbidity and mortality in people with type 1 diabetes.18 A recent, large database study from Sweden found that, while mortality rates have been falling in patients with type 1 diabetes since the turn of the century, the incidence of CV death has remained ~ 5‐fold higher compared with controls matched for age, gender and location.19 A database study from Scotland, UK, showed that the excess CV risk begins early in life: patients with type 1 diabetes aged 20–39 years had an ~ 5‐fold higher incidence of a first CV event compared with the non‐diabetic population.20 In another study, patients with type 1 diabetes at 20 years of age were found to face, on average, a loss of 11 years (men) or 13 years (women) of life, compared with the background population, and CV disease accounted for the majority of this early mortality.21
The REMOVAL trial was designed to evaluate the CV effects of metformin in a population of 428 middle‐aged (≥40 years, mean age at baseline 55 years) people with type 1 diabetes of ≥5 years duration (mean 33 years), who had suboptimal glycaemic control (HbA1c >7.0%, mean HbA1c 8.0%), with at least three of a list of 10 prespecified CV risk factors.22 This was clearly a population at high CV risk, as 78% were overweight or obese (mean body mass index 28.4 kg/m2), 12% had prior CV disease, 13% were smokers, 15% had microalbuminuria and a substantial proportion received treatment with a statin (82%), an antihypertensive (73%) or an antiplatelet agent (39%). A 3‐month run‐in phase to optimize insulin dosage and CV risk factors was followed by randomization to metformin (target dose 2000 mg/day) or placebo for 3 years, each in addition to standard of care for type 1 diabetes. The primary outcome was mean far‐wall carotid intima‐media thickness (cIMT, a surrogate measure of the overall burden of atherosclerosis)23 measured annually.
Modest, but significant, reductions occurred over 3 years in the metformin versus placebo group in HbA1c (mean treatment difference −0.13%, P = 0.006), bodyweight (−1.17 kg, P < 0.0001) and LDL cholesterol (−0.13 mmol/L, P = 0.0017). The primary endpoint was not met (mean change vs. placebo in far‐wall cIMT was −0·005 mm/year [−0·012 to 0·002], P = 0.1664), and so the overall outcome of the trial was neutral. However, metformin significantly reduced mean change/year in maximal cIMT, a prespecified tertiary endpoint (−0·013 mm/year [−0·024 to −0·003] vs. placebo, P = 0.0093).
2.4. Observational studies and meta‐analyses
We have concentrated on evidence from randomized trials in this article, as observational data are subject to confounding and are too extensive to describe in detail here. In addition, larger observational studies of metformin (ie, with data from at least 1000 subjects) have been reviewed elsewhere.5 Statistically and clinically significant reductions in adverse CV outcomes have been observed in many of these studies, whether metformin was compared with no treatment, lifestyle intervention or SU. In addition, observational data indicated clinical benefit of metformin in subjects with reduced renal function and congestive heart failure.
Meta‐analyses provide conflicting conclusions on whether metformin reduces the risk of adverse CV outcomes.24, 25, 26 The availability of long‐term evaluations of metformin is limited, which hinders effective meta‐analysis. For example, one recent meta‐analysis of the effect of metformin on the incidence of MI included seven trials, with durations ranging from 6 months to 10 years, and with numbers of events ranging from 14 to 423.24
A recent, very large meta‐analysis included more than 1 million patients, who participated in 40 randomized or observational evaluations of metformin.27 Treatment with metformin versus no metformin therapy was associated with reduced risk of CV death (adjusted HR 0.81 [95% CI 0.79 to 0.84]). Moreover, all‐cause mortality was reduced in the overall population (adjusted HR 0.67 [95% CI 0.60 to 0.75]), in those with prior MI (adjusted HR 0.79 [95% CI 0.68 to 0.92]), and in those with prior congestive heart failure (adjusted HR 0.84 [95% CI 0.81 to 0.87]). The frequency of CV events was also reduced, although interestingly no significant effect was observed in the absence of type 2 diabetes.
3. METFORMIN IN CHRONIC KIDNEY DISEASE
3.1. Kidney disease markedly increases CV risk in patients with type 2 diabetes
A recent study in 687 732 patients with type 2 diabetes showed that 18% had a first adverse cardiorenal event during 4 years of follow‐up.28 Chronic kidney disease (CKD) accounted for 36% of these events, and was the earliest and most common CV complication of diabetes in this population. Moreover, the appearance of CKD was associated with a 1.8‐fold increase in the risk of all‐cause or CV death. Other data from the nationally representative US National Health and Nutrition Examination Survey (NHANES) confirm the severe adverse impact of a diagnosis of CKD on clinical outcomes in people with diabetes.29 In this cohort, the age‐standardized mortality rate was only 4.1% higher for people with type 2 diabetes without CKD than for controls without either condition. However, the difference from the control group in age‐standardized mortality increased by 17.8% where albuminuria was present, by 23.9% where glomerular filtration rate (GFR) was abnormal, and by 47.0% where subjects had both albuminuria and abnormal GFR. Thus, the excess mortality associated with diabetes was mostly accounted for by people with CKD.
3.2. Renal safety of metformin
Intensive glycaemic control per se is widely considered to reduce the risk of end‐stage renal disease (ESRD) in type 2 diabetes, largely on the basis of a 65% reduction in this outcome in patients randomized to intensive versus standard blood glucose control policies in the ADVANCE trial (HR 0.35, 95% CI 0.15 to 0.83, P = 0.01).30 However, it should be noted that the confidence intervals are wide, as even in this large trial with 11 140 patients randomized, there were only 27 events over 5 years of follow‐up.30
Metformin was contraindicated in people with CKD (estimated GFR [eGFR] <60 mL/min/1.73m2) for many years, however, with the intention of mitigating a perceived association between the use of metformin and an increased risk of lactic acidosis. Metformin increases serum lactate levels modestly with serum lactate concentrations usually remaining well within the normal range.31 However, as metformin is excreted unchanged via the kidney,32 a cautious approach to labelling of metformin has been adopted in the setting of CKD by international regulators in order to reduce the potential for accumulation and the possibility of developing lactic acidosis.
The current European labelling for metformin specifies that this agent is contraindicated in patients with eGFR below 30 mL/min/1.73m2.33 Prospective data are now available on the efficacy and safety of metformin in people with CKD from three complementary studies carried out in people with type 2 diabetes and CKD.34 Briefly, study 1 involved short‐term dose titration (1 week) of metformin 500–2000 mg/day in 78 patients with CKD 1–5; study 2 involved 4 months of administration of metformin 1500 mg/day to 46 patients with CKD 3–4; and study 3 was a detailed pharmacokinetic and pharmacodynamic study of metformin in patients with CKD 3–4. Plasma metformin was measured 12 hours after administration (24 hours for people with CKD 4).
Marked elevations of plasma metformin were rare in study 1, and hyperlactataemia did not occur (summarized in Table 1). Examination of serum metformin concentrations at different CKD stages in study 1 enabled selection of appropriate dose levels for use in study 2 (1500, 1000 and 500 mg/day for patients with CKD 3A, 3B and 4, respectively). During 4 months of metformin treatment, plasma metformin levels remained consistently below 2.5 mg/L in most patients (Figure 3). The pharmacokinetics of metformin changed little across CKD stages 3A‐4.
Table 1.
Overview of results from studies of the safety of metformin in patients with different severities of chronic kidney disease34
| Plasma metformin concentrations correlated inversely with glomerular filtration rate, as would be expected given the exclusively renal elimination of metformin. |
| “Moderately elevated” serum metformin concentrations (>2.5 mg/L) were observed in: |
| 1/75 subjects at 500 mg/day |
| 5/74 subjects at 1000 mg/day |
| 17/68 subjects at 2000 mg/day. |
| “Clearly elevated” serum metformin levels (>5 mg/L) were observed in |
| 1/74 patients receiving metformin 1000 mg/day |
| 2/68 patients receiving metformin 2000 mg/day. |
| Hyperlactataemia (serum lactate >5 mmol/L) was not observed |
| Only one patient had two consecutive lactate levels >2.5 mmol/L. |
Figure 3.
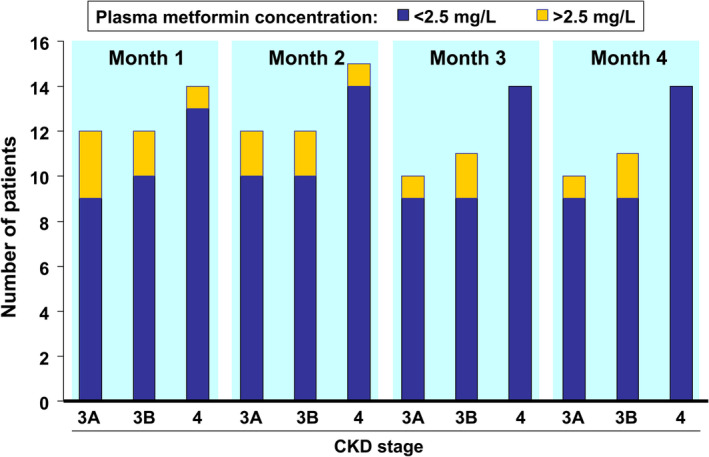
Serum concentrations of metformin and lactate above and below 2.5 mg/L during 4 months of administration of metformin to people with type 2 diabetes and chronic kidney disease (CKD). Total daily metformin dosages differed between patients with CKD 3A (1500 mg), CKD 3B (1000 mg) and CKD 4 (500 mg); see text. Drawn from data presented in reference34
The Comparative Outcomes Study of Metformin Intervention versus Conventional (COSMIC) Approach Study, a randomized evaluation of metformin (n = 7207) versus other usual care glucose‐lowering therapies (n = 1505), also reported no cases of lactic acidosis during 1 year of follow‐up.35
Given the paucity of randomized trial evidence on renal outcomes beyond the study described above, data from key observational studies are also of interest. A study based on a community‐based cohort of 75 513 subjects in Germany who were followed from 2004 to 2017 showed that there was a significant association between metformin use and lactic acidosis for patients with eGFR <30 mL/min/1.73m2 (adjusted HR 2.07 [1.33 to 3.22]), but not in those with eGFR 45–59 mL/min/1.73m2 (adjusted HR 1.16 [0.95 to 1.41]) or eGFR 30–44 mL/min/1.73m2 (adjusted HR 1.09 [0.83 to 1.44]).36 These findings were similar in a second study using a cohort of new users of metformin and SU from the same health system.36 A further validation using propensity score‐matched data from a commercial US database found no association between lactic acidosis and metformin (vs. SU comparator) at any level of eGFR.36
Lactic acidosis is an acutely life‐threatening event, and it is clearly important to minimize its occurrence. The risk of lactic acidosis when metformin is prescribed appropriately is low, and patients with “metformin associated lactic acidosis” often have limited documentation of the circulating level of metformin, or have additional risk factors for lactic acidosis.37, 38 The studies described above suggested that treatment with metformin is effective and safe, with appropriate dose adjustment, for people with CKD. Overall, the current limitation on the use of metformin to patients with eGFR >30 mL/min/1.73m2, with appropriate caution for people with eGFR 30–45 mL/min/1.73m2, appears to be a pragmatic solution to ensuring the renal safety of metformin‐based therapy. Educating prescribers and metformin‐treated patients with CKD to follow “sick day rules” to temporarily suspend intake of metformin during acute illness, especially an episode of acute kidney injury, is also important, as acute kidney injury or other acute, severe illnesses can markedly increase lactate levels.39, 40
Real‐world data suggest the possibility of CV benefit with metformin in populations with CKD. A recent retrospective analysis of propensity score‐matched cohorts of 67 749 patients receiving metformin and 28 976 receiving SU displayed a significant reduction in the risk of MACE, with adjusted HR 0.80 (95% CI 0.75 to 0.86).41 Another observational study consisted of 508 propensity score‐matched pairs of patients receiving or not receiving metformin taken from the population of a randomized, controlled evaluation of darbepoetin alfa (to address anaemia) in patients with type 2 diabetes and CKD (mean estimated eGFR at baseline was 33 mL/min/1.73m2).42 This event‐driven trial concluded after a median follow‐up duration of 29 months with a neutral outcome for the study therapy. However, use versus non‐use of metformin was associated with a reduced risk of CV death (HR 0.49 [0.32 to 0.74]), a composite CV endpoint (death, heart failure hospitalization, MI, stroke or myocardial ischaemia; HR 0.67 [0.51 to 0.88]), a composite of renal outcomes (ESRD or death; HR 0.77 [0.61 to 0.98]) and all‐cause death (HR 0.49 [0.36 to 0.69]). There was no increased risk of ESRD with metformin (HR 1.01 [0.65 to 1.55]).
3.3. Potential for renal protective effects of metformin
As clinical data on renal outcomes in metformin‐treated patients are limited, this section will review available data from people with diabetes, and will then consider experimental work in this area, including observational data where needed. The results of such observational studies come lower in the hierarchy of evidence than those from randomized trials, but can provide additional insights where RCT data are lacking.43
There was no significant effect of metformin versus the diet‐based control treatment on renal death or renal failure in the UKPDS, but there were only six events of this type recorded in this newly diagnosed population.7 The REMOVAL trial in people with type 1 diabetes recorded no instances of eGFR declining to <30 mL/min/1.73m2 in either study arm.22 However, eGFR was better maintained over time in the metformin group, with a between‐group difference of 4.0 mL/min/1.73m2 (2.19 to 5.81, P < 0.001) in favour of metformin over 3 years.22
In the propensity‐score substudy within the darbopoetin RCT described above, the incidence of two renal outcomes was reduced in the metformin group: a renal composite endpoint (P = 0.02) and this endpoint plus doubling of serum creatinine (P = 0.03).42 These findings may have been driven mainly by the effect of metformin on mortality, as the analysis was neutral for ESRD as an individual outcome. A study of 24 158 matched pairs of metformin users and non‐users from the Taiwan National Health Insurance database suggested a small but significant increase in the risk of ESRD with metformin.44 Interestingly, in a recent subgroup analysis of the EMPA‐REG Outcome trial, it was suggested that renoprotection with empagliflozin might be attenuated by background metformin therapy on the basis that there was a non‐significant trend (P for interaction = 0.07) towards higher hazard ratios for nephropathy events with concomitant metformin therapy (0.68 [95% CI 0.58 to 0.79]) compared with no metformin therapy (0.47 [95% CI 0.37 to 0.59]).45 However, this appears to be a misinterpretation of the data: absolute rates of incident or worsening nephropathy were lower overall in those on metformin (12.4% with empagliflozin vs. 16.9% on placebo) than in those who were not on metformin (13.7% with empagliflozin vs. 24.6% on placebo), ie, additional renoprotection with empagliflozin may have been more difficult to detect in participants of this trial, for whom metformin was already providing background renoprotection.
More clinical data on appropriate composite renal outcomes from large RCTs are required in the light of these conflicting data. The Metformin as RenoProtector of Progressive Kidney Disease trial (RenoMet) is evaluating metformin versus placebo in people without any type of diabetes who have CKD 2‐3B (eGFR 30–90 mL/min/1.73m2), with a history of annual decline in eGFR of 2–15 mL/min/1.73m2 over the preceding 3 years, and proteinuria ≤2 g/24 hours.46 The primary outcome is time to 30% decline in eGFR. RenoMet is expected to complete by the end of 2021 and will be informative with regard to the effects of metformin on cardiorenal outcomes.
4. DISCUSSION AND PERSPECTIVES
4.1. Clinical outcomes trials in diabetes: then and now
The contemporary era of CVOTs was prompted initially by concerns over drug safety, following the publication in 2007 of a meta‐analysis of trials of a thiazolidinedione glucose‐lowering agent, rosiglitazone that suggested a significant increase in the risk of mortality (43%, P = 0.03) and a barely non‐significant increase in the risk of CV death (64%, P = 0.06), relative to non‐thiazolidinedione comparators.47 These findings were not ultimately substantiated,48 but nevertheless highlighted a lack of oversight of CV safety within the development of new therapies for type 2 diabetes.49 In 2008, the US Food and Drug Administration (FDA) introduced new guidelines for the CV evaluation of glucose‐lowering treatments.50
Thus, we can divide the history of CVOTs in diabetes into two periods. Before 2008, the evaluation of clinical outcomes in diabetes was performed rarely, and individual trials were designed without specific reference to the design of other trials. After 2008, we have seen numerous randomized, (almost all) placebo‐controlled, outcome trials, usually with primary outcomes of, or similar to, “three‐point” or “four point” MACE outcomes.51 Caution must always be exercised in comparing the results of one trial directly with another, but these studies can be interpreted as showing that GLP‐1 receptor agonists act mainly against atherosclerotic disease to improve outcomes, while SGLT2 inhibitors may be especially effective in reducing the risk of endpoints related to heart failure and/or renal dysfunction.52, 53 It is important to note that ~ 75% of patients randomized within recent CVOTs of SGLT2 inhibitors or GLP‐1 receptor agonists were already receiving metformin at baseline.54
Recently updated guidance from the European Society of Cardiology ([ESC] in collaboration with representatives of the EASD) recommends that one of these agents should be prescribed irrespective of HbA1c for patients with CV disease, or who are at high or very high CV risk, especially where the risk of heart failure is elevated.55 Drug‐naïve patients could receive one of these antidiabetic agents as initial glucose‐lowering pharmacotherapy, in contrast to current transatlantic diabetes guidelines (EASD‐ADA consensus) which continue to recommend metformin as first pharmacologic treatment.2 The ESC recommendations have proved controversial, not least because of the increased healthcare costs implied by adding expensive, branded medications to the comparatively inexpensive treatment, metformin.56 There is a need for new, well‐designed clinical trials comparing metformin with newer agents.
But how do we consider the older generation of glucose‐lowering agents that have not had the benefit of this intensive, FDA‐mandated evaluation of outcomes? The concept of CV protection with metformin is supported by three RCTs in patients with type 2 diabetes, which cover the natural history of diabetes from the newly diagnosed population of the UKPDS with a low prevalence of CV complications at baseline,7, 57 to patients with type 2 diabetes who already have coronary artery disease,13 to patients with type 2 diabetes who have spent some years taking insulin.12 Moreover, data from other populations, such as people with type 1 diabetes,22, 58 support the presence of antiatherosclerotic mechanisms of metformin.
The randomized trials that evaluated metformin were small by current trial standards, although the 10‐year UKPDS employed a considerably longer follow‐up duration than most other studies. The Kaplan–Meier curves for macrovascular outcomes in the metformin and conventional therapy groups did not diverge until about 6 years into the study.7 Larger studies, such as ADOPT or COSMIC (which were not designed as outcomes trials), were of shorter duration, insufficient to show benefit. Obtaining more, and more definitive, clinical data is always a useful answer to questions like these, and the ongoing VA‐IMPACT outcomes trial in subjects with prediabetes (even earlier in the disease course of type 2 diabetes than the UKPDS) will provide valuable additional data (expected to report in mid‐2024).59 Table 2 summarizes the patient population, design and outcomes of this trial.59
Table 2.
Overview of the ongoing VA‐IMPACT trial (NCT02915198)
| Title: | Investigation of Metformin in Prediabetes on Atherosclerotic Cardiovascular OuTcomes (VA‐IMPACT) |
|---|---|
| Design: | Multicentre, prospective, randomized, double‐blind |
| Patients: | Required to have: |
|
Prediabetes: HbA1c ≥5.7%, <6.5% (≥39, <48 mmol/mol) and/or Fasting plasma glucose 100–125 mg/dL (5.6–6.9 mmol/L) and/or 2 h post‐load glucose 140–199 mg/dL (7.8–11.1 mmol/L) after a 75 g oral glucose tolerance test |
|
| Pre‐existing cardiovascular or cerebrovascular disease | |
| Key exclusions: | Glucose‐lowering therapy |
| Estimated glomerular filtration rate <45 mL/min/1.73m2 | |
| Known intolerance to metformin | |
| Pregnancy, planning pregnancy or lactating | |
| Treatments: | Metformin XR (up to 2000 mg/day) |
| Placebo | |
| Outcomes: | Primary: Death, non‐fatal myocardial infarction (MI), stroke, hospitalization for unstable angina, or symptom‐driven coronary revascularization |
| Secondary: Death, MI or stroke | |
| Primary endpoint, peripheral arterial disease event or hospitalization for congestive heart failure | |
| Incidence of all components of the primary endpoint including recurrent or multiple events in the same participant | |
| Each component of the primary outcome measure, peripheral arterial disease events and hospitalization for congestive heart failure | |
| New or recurrent malignancy or death from a malignancy | |
| New diagnosis of type 2 diabetes (American Diabetes Association criteria) |
Note: Compiled from information presented in reference.59
4.2. What mechanisms could account for CV protection with metformin?
Numerous mechanisms have been proposed to account for the observations of cardioprotective effects with metformin described above. A detailed description of these is beyond the scope of our article, and they have been reviewed elsewhere and are summarized in Figure 4.60, 61, 62, 63, 64, 65 Briefly, RCTs or systematic reviews have shown improvements in endothelial function66 and fibrinolysis67, 68, 69 in metformin‐treated patients that are consistent with reduced CV risk. Effects on lipid profiles are modest, but have included reductions in LDL cholesterol and triglycerides.70, 71 Numerous findings from experimental systems have been described, including direct cellular antiatherogenic effects (on vascular cells and cholesterol‐depositing macrophages), improved microcirculation, reduced oxidative stress (including that associated with deposition of advanced glycation end products [glycoxidation]), reduced markers of inflammation (including in patients with type 1 diabetes in the REMOVAL trial),67 effects on the immune system, and modulation of the gut microbiome.60, 61, 62, 63, 64, 65 In addition, a recently published study randomized 37 non‐diabetic patients with heart failure to metformin (mean dose 1450 mg/day) or to placebo for 3 months, in addition to guideline‐driven, maximally tolerated pharmacologic treatment for heart failure.72 Randomization to metformin was associated with improved myocardial efficiency, with reduced myocardial oxygen consumption. While this population did not have diabetes, the authors noted that insulin resistance plays an important part in the cardiac pathophysiology of heart failure.
Figure 4.
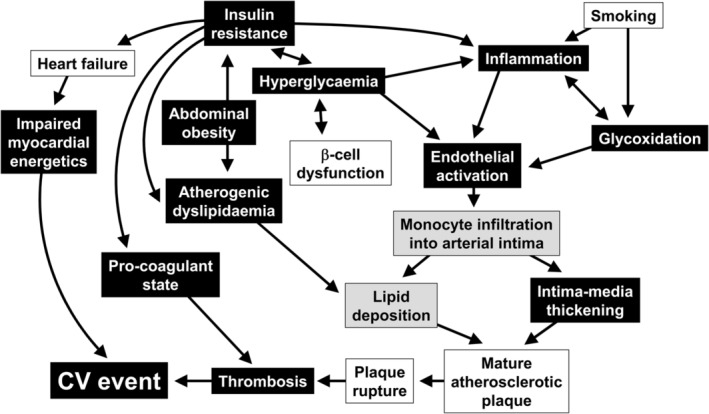
Proposed antiatherothrombotic mechanisms of metformin. Effects observed in humans are shown in black rectangles, while effects shown in experimental studies are shown in shaded rectangles. Adapted from reference65 with permission from Elsevier; see also references in this review
It is unclear whether weight loss on metformin contributes to CV protection: although uncontrolled studies have described weight loss on this agent, metformin was weight‐neutral overall in several randomized trials (reviewed elsewhere).73 One study (published in abstract form only) reported a reduction in visceral adipose tissue with metformin versus placebo.74 Finally, metformin has been observed to increase secretion of GLP‐1. It is unclear whether this is in itself a cardioprotective mechanism; however, post hoc analysis of three CVOTs of dipeptidyl peptidase‐4 (DPP‐4) inhibitors (which protect GLP‐1 from enzymic degradation) indicates numerically (but not statistically) lower rates of CV outcomes when DPP‐4 inhibitors are added to metformin, and (numerically) higher rates when a DPP‐4 inhibitor is taken without metformin.75 This is a hypothesis‐generating observation that requires further study.
Experimental studies are increasing understanding of the potential renoprotective effects of metformin. A recent review highlighted potentially beneficial renal mechanisms of metformin, especially signalling through the AMP kinase (AMPK)/mTOR pathway, reduction of endoplasmic reticulum stress, modulation of endothelial to mesenchymal transition, promotion of autophagy to clear away damaged cells and organelles, inhibition of reactive oxygen species and inflammation and oxidation caused by advanced glycation end products, and reduced lipoxicity.76, 77 A decline in the activity of AMPK parallels the development of structural and functional abnormalities in the kidneys of mice with experimental CKD, and metformin treatment has been shown to reverse early changes of this type by restoring AMPK activity.78 Reduced expression of the SHIP2 gene by metformin is another candidate mechanism.79 This gene is overexpressed in animal models of diabetes, in which it is associated with insulin resistance and reduced glucose transport.80 SHIP2 is also upregulated in the glomeruli of patients with type 2 diabetes, but not in patients receiving metformin, and metformin treatment has been associated observationally with reduced loss of renal podocytes in patients with type 2 diabetes.80, 81
5. CONCLUSIONS
The whole field of clinical trials in diabetes has changed dramatically since 2008, leading to a number of evaluations of newer glucose‐lowering therapies that are comparatively homogenous in design, and amenable to meta‐analysis. Accordingly, it is difficult to compare the actions of an older therapy such as metformin with the results of these recent trials. Nevertheless a variety of lines of evidence exist to support an antiatherosclerotic effect of metformin in people with diabetes and a reduced risk of adverse CV outcomes in type 2 diabetes. Although those data were collected in an earlier era of clinical trial design, they must be taken into account in any balanced view of the evidence. Recent evidence from clinical trials has shown that metformin is effective and safe in people with CKD, as long as appropriate dose adjustments are used, and use is restricted to patients with eGFR >30 mL/min/1.73m2. Although we have now seen more than 6 decades of continuous therapeutic use of metformin, further data that will better define its cardioprotective and renoprotective effects and mechanisms of metformin are set to emerge over the next 5 years.
CONFLICT OF INTEREST
I.W.C. has received honoraria for work on advisory boards and lecturing from Johnson & Johnson, Lilly, Merck, MSD, Sanofi and Takeda. J.R.P. has received payment via the University of Glasgow for advisory and consultancy work on behalf of Novo Nordisk, Sanofi‐Aventis and for work on clinical trials committees on behalf of ACI Clinical (Boehringer), Janssen, Novo Nordisk, Quintiles (Genentech, Roche), and Sanofi‐Aventis, and has received travel and accommodation support from Novo Nordisk and Merck KGaA. J.R.P. has also received donation of services to support investigator‐led research from Merck KGaA, Itamar Medical (Israel) and DEXCOM, and investigator‐led research funding from Janssen (RISC study). J.R.P. holds no stock in any pharmaceutical or technology company. P.R.R. has received consultancy and/or speaking fees (to his institution) from AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Gilead, Eli Lilly, MSD, Merck, Mundi, Novo Nordisk and Sanofi Aventis, and has received research grants from AstraZeneca and Novo Nordisk.
AUTHOR CONTRIBUTIONS
All authors contributed actively to the development of the article, suggested material for inclusion, reviewed the article as it developed, and approved the final version for submission.
ACKNOWLEDGMENTS
A medical writer (Dr Mike Gwilt, GT Communications, funded by Merck Healthcare KGaA) provided editorial assistance to the authors, who retained full control of the article throughout.
This article was inspired by a satellite symposium held on the occasion of the 55th Annual Meeting of the European Association for the Study of Diabetes, sponsored by Merck Healthcare KGaA, at which the authors presented. The sponsor of this meeting had no role in the development of this article.
Petrie JR, Rossing PR, Campbell IW. Metformin and cardiorenal outcomes in diabetes: A reappraisal. Diabetes Obes Metab. 2020;22:904–915. 10.1111/dom.13984
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13984.
Funding information Merck; European Association for the Study of Diabetes; Food and Drug Administration
REFERENCES
- 1. Nagahisa T, Saisho Y. Cardiorenal protection: potential of SGLT2 inhibitors and GLP‐1 receptor agonists in the treatment of type 2 diabetes. Diabetes Ther. 2019;10:1733‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pratley RE, Handelsman Y. Should metformin be the first‐line therapy choice in type 2 diabetes treatment? Endocrine Today. https://www.healio.com/endocrinology/diabetes/news/print/endocrine-today/%7B0d91ed6f-9d3e-4cbd-908d-5d4802b65a24%7D/should-metformin-be-the-first-line-therapy-choice-in-type-2-diabetes-treatment. Accessed October, 2019.
- 4. Bailey CJ, Campbell IW, JCN C, Davidson JA, HCS H, Ritz P, eds. Metformin: The Gold Standard. A Scientific Handbook. Chichester, UK: Wiley; 2007. [Google Scholar]
- 5. Campbell IW, HCS H, Holman RR, Bailey CJ, eds. Metformin: 60 years of Clinical Experience. Addendum to the Scientific Handbook. Weinheim, Germany: Wiley; 2016. [Google Scholar]
- 6. Marshall SM. 60 years of metformin use: a glance at the past and a look to the future. Diabetologia. 2017;60:1561‐1565. [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854‐865. [PubMed] [Google Scholar]
- 8. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837‐853. [PubMed] [Google Scholar]
- 9. UKPDS . Correspondence. Lancet. 1998;352:1932‐1934. [Google Scholar]
- 10. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 11. Holman RR. UKPDS: 15 years on from Barcelona. Post trial monitoring results of the UKPDS sulfonylurea plus metformin substudy. Presentation at the 49th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, 23–27 September, 2013. https://www.easd.org/virtualmeeting/home.html#!resources/post-trial-monitoring-results-of-the-ukpds-sulfonylurea-plus-metformin-substudy. Accessed January, 2020. [Google Scholar]
- 12. Kooy A, de Jager J, Lehert P, et al. Long‐term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616‐625. [DOI] [PubMed] [Google Scholar]
- 13. Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427‐2443. [DOI] [PubMed] [Google Scholar]
- 15.Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). https://grade.bsc.gwu.edu. Accessed January, 2020.
- 16. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wexler DJ, Krause‐Steinrauf H, Crandall JP, et al. Baseline characteristics of randomized participants in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2019;42:2098‐2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrie JR, Sattar N. Excess cardiovascular risk in type 1 diabetes mellitus. Circulation. 2019;139:744‐747. [DOI] [PubMed] [Google Scholar]
- 19. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407‐1418. [DOI] [PubMed] [Google Scholar]
- 20. Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008‐2010. JAMA. 2015;313:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petrie JR, Chaturvedi N, Ford I, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double‐blind, randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2017;5:597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bots ML, Grobbee DE. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther. 2002;16:341‐351. [DOI] [PubMed] [Google Scholar]
- 24. Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta‐analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60:1620‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2011;13:221‐228. [DOI] [PubMed] [Google Scholar]
- 26. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all‐cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta‐analysis. Ageing Res Rev. 2017;40:31‐44. [DOI] [PubMed] [Google Scholar]
- 27. Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all‐cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta‐analysis. Cardiovasc Diabetol. 2019;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Birkeland K, Bodegard J, Eriksson J, et al. Cardiorenal disease is the most common first CV manifestation in type 2 diabetes and associated with increased mortality: a large multinational observational study. Abstract/presentation 126 at the 55th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 16–20, September 2019. https://www.easd.org/virtualmeeting/home.html#!resources/cardiorenal-disease-is-the-most-common-first-cv-manifestation-in-type-2-diabetes-and-associated-with-increased-mortality-a-large-multinational-observational-study. Accessed October, 2019. [Google Scholar]
- 29. Afkarian M1, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517‐523. [DOI] [PubMed] [Google Scholar]
- 31. Huang W, Castelino RL, Peterson GM. Lactate levels with chronic metformin use: a narrative review. Clin Drug Investig. 2017;37:991‐1007. [DOI] [PubMed] [Google Scholar]
- 32. Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81‐98. [DOI] [PubMed] [Google Scholar]
- 33.European Medicines Agency. Metformin and metformin‐containing medicines. https://www.ema.europa.eu/en/medicines/human/referrals/metformin-metformin-containing-medicines. Accessed January, 2020.
- 34. Lalau JD, Kajbaf F, Bennis Y, Hurtel‐Lemaire AS, Belpaire F, de Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41:547‐553. [DOI] [PubMed] [Google Scholar]
- 35. Cryer DR, Nicholas SP, Henry DH, Mills DJ, Stadel BV. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care. 2005;28:539‐543. [DOI] [PubMed] [Google Scholar]
- 36. Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community‐based cohort study. JAMA Intern Med. 2018;178:903‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lalau JD, Kajbaf F, Protti A, Christensen MM, De Broe ME, Wiernsperger N. Metformin‐associated lactic acidosis (MALA): Moving towards a new paradigm. Diabetes Obes Metab. 2017;19:1502‐1512. [DOI] [PubMed] [Google Scholar]
- 38. Bicsak TA, Walsh B, Fineman M. Metformin‐associated lactic acidosis: Moving towards a new paradigm? Diabetes Obes Metab. 2017;19:1499‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. MacCallum L, Senior PA. Safe use of metformin in adults with type 2 diabetes and chronic kidney disease: lower dosages and sick‐day education are essential. Can J Diabetes. 2019;43:76‐80. [DOI] [PubMed] [Google Scholar]
- 40. Connelly PJ, Lonergan M, Soto‐Pedre E, Donnelly L, Zhou K, Pearson ER. Acute kidney injury, plasma lactate concentrations and lactic acidosis in metformin users: A GoDarts study. Diabetes Obes Metab. 2017;19:1579‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roumie CL, Chipman J, Min JY, et al. Association of treatment with metformin vs sulfonylurea with major adverse cardiovascular events among patients with diabetes and reduced kidney function. JAMA. 2019;1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charytan DM, Solomon SD, Ivanovich P, et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019;21:1199‐1208. [DOI] [PubMed] [Google Scholar]
- 43. Sahay BK, Seshiah V. Importance of observational studies in understanding regional clinical practice: rationale and design of the A1chieve study. J Assoc Physicians India. 2013;61(1 Suppl):6‐8. [PubMed] [Google Scholar]
- 44. Lee MC, Lee CH, Chang LY, et al. Association of metformin use with end‐stage renal disease in patients with type 2 diabetes mellitus: a nationwide cohort study under the Pay‐for‐Performance Program. J Clin Pharmacol. 2019;59:1443‐1452. [DOI] [PubMed] [Google Scholar]
- 45. Inzucchi SE, Fitchett D, Jurišić‐Eržen D, et al. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose‐lowering therapy? [published online ahead of print December 2, 2019]. Diabetes Obes Metab. https://doi: 10.1111/dom.13938. [DOI] [PubMed] [Google Scholar]
- 46. ClinicalTrials.gov. Metformin as RenoProtector of Progressive Kidney Disease (RenoMet). ClinicalTrials.gov Identifier: NCT03831464. https://clinicaltrials.gov/ct2/show/record/NCT03831464. Accessed January, 2020.
- 47. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457‐2471. [DOI] [PubMed] [Google Scholar]
- 48. Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs–insights from the rosiglitazone experience. N Engl J Med. 2013;369:1285‐1287. [DOI] [PubMed] [Google Scholar]
- 49. Liebson PR. The rosiglitazone controversy: meta‐analysis and the RECORD study. Prev Cardiol. 2007;10:235‐237. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry. Diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. December 2008. https://www.fda.gov/media/71297/download. Accessed October, 2019.
- 51. Marx N, McGuire DK, Perkovic V, et al. Composite primary end points in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. 2017;40:1144‐1151. [DOI] [PubMed] [Google Scholar]
- 52. Hupfeld C, Mudaliar S. Navigating the "MACE" in cardiovascular outcomes trials and decoding the relevance of atherosclerotic cardiovascular disease benefits versus heart failure benefits. Diabetes Obes Metab. 2019;21:1780‐1789. [DOI] [PubMed] [Google Scholar]
- 53. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 54. American Diabetes Association. 10 . Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes‐2020. Diabetes Care. 2020;43(Suppl 1):S111‐S134. [DOI] [PubMed] [Google Scholar]
- 55. Cosentino F, Grant PJ, Aboyans V, et al. ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 56. Jorsal A, Persson F, Bruun JM. Comments on the 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 57. UKPDS Study Group . UK Prospective Diabetes Study 6. Complications in newly diagnosed type 2 diabetic patients and their association with different clinical and biochemical risk factors. Diabetes Res. 1990;13:1‐11. [PubMed] [Google Scholar]
- 58. Livingstone R, Boyle JG, Petrie JR, REMOVAL Study Team . A new perspective on metformin therapy in type 1 diabetes. Diabetologia. 2017;60:1594‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. ClinicalTrials.gov. Investigation of Metformin in Pre‐Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA‐IMPACT). https://clinicaltrials.gov/ct2/show/record/NCT02915198. Accessed January, 2020.
- 60. Zilov AV, Abdelaziz SI, AlShammary A, et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab Res Rev. 2019;35:e3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jenkins AJ, Welsh P, Petrie JR. Metformin, lipids and atherosclerosis prevention. Curr Opin Lipidol. 2018;29:346‐353. [DOI] [PubMed] [Google Scholar]
- 63. Pollak M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia. 2017;60:1662‐1667. [DOI] [PubMed] [Google Scholar]
- 64. McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chan JC, Deerochanawong C, Shera AS, et al. Role of metformin in the initiation of pharmacotherapy for type 2 diabetes: an Asian‐Pacific perspective. Diabetes Res Clin Pract. 2007;75:255‐266. [DOI] [PubMed] [Google Scholar]
- 66. De Jager J, Kooy A, Lehert P, et al. Effects of short‐term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo‐controlled trial. J Intern Med. 2005;257:100‐109. [DOI] [PubMed] [Google Scholar]
- 67. Welsh P, Greenlaw N, Colhoun H, et al. The REMOVAL Trial: Effect of metformin on markers of cardiometabolic risk in patients with type 1 diabetes. Abstract/poster 465‐P at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, USA, June 7–11, 2019. https://ada.scientificposters.com/epsAbstractADA.cfm?id=1. Accessed October, 2019. [Google Scholar]
- 68. Grant PJ, Stickland MH, Booth NA, Prentice CR. Metformin causes a reduction in basal and post‐venous occlusion plasminogen activator inhibitor‐1 in type 2 diabetic patients. Diabet Med. 1991;8:361‐365. [DOI] [PubMed] [Google Scholar]
- 69. Grant PJ. The effects of high‐ and medium‐dose metformin therapy on cardiovascular risk factors in patients with type II diabetes. Diabetes Care. 1996;19:64‐66. [DOI] [PubMed] [Google Scholar]
- 70. Wulffele MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med. 2004;256:1‐14. [DOI] [PubMed] [Google Scholar]
- 71. Saenz A, Fernandez‐Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;9:CD002966. [DOI] [PubMed] [Google Scholar]
- 72. Larsen AH, Jessen N, Nørrelund H, et al. A randomised, double‐blind, placebo‐controlled trial of metformin on myocardial efficiency in insulin‐resistant chronic heart failure patients without diabetes [published online ahead of print December 21 , 2019]. Eur J Heart Fail . https://doi: 10.1002/ejhf.1656. [DOI] [PubMed]
- 73. Golay A. Metformin and body weight. Int J Obes (Lond). 2008;32:61‐72. [DOI] [PubMed] [Google Scholar]
- 74. Kurukulasuriya R, Banerji MA, Chaiken R, Lebovitz H. Selective decrease in visceral fat is associated with weight loss during metformin treatment in African Americans with type 2 diabetes. Diabetes. 1999;48(Suppl):A315. [Google Scholar]
- 75. Crowley MJ, Williams JW Jr, Kosinski AS, D'Alessio DA, Buse JB. Metformin use may moderate the effect of DPP‐4 inhibitors on cardiovascular outcomes. Diabetes Care. 2017;40:1787‐1789. [DOI] [PubMed] [Google Scholar]
- 76. Ravindran S, Kuruvilla V, Wilbur K, Munusamy S. Nephroprotective effects of metformin in diabetic nephropathy. J Cell Physiol. 2017;232:731‐742. [DOI] [PubMed] [Google Scholar]
- 77. Corremans R, Vervaet BA, D'Haese PC, Neven E, Verhulst A. Metformin: a candidate drug for renal diseases. Int J Mol Sci. 2018;20:E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Satriano J, Sharma K, Blantz RC, Deng A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F727‐F733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lehtonen S. SHIPping out diabetes‐Metformin, an old friend among new SHIP2 inhibitors. Acta Physiol. 2019;228:e13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Polianskyte‐Prause Z, Tolvanen TA, Lindfors S, et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J. 2019;33:2858‐2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hyvönen ME, Saurus P, Wasik A, et al. Lipid phosphatase SHIP2 downregulates insulin signalling in podocytes. Mol Cell Endocrinol. 2010;328:70‐79. [DOI] [PubMed] [Google Scholar]


