The treatment of dogs naturally infected with Leishmania infantum using meglumine antimoniate (MA) encapsulated in conventional liposomes (LC) in association with allopurinol has been previously reported to promote a marked reduction in the parasite burden in the main infection sites. Here, a new assay in naturally infected dogs was performed using a novel liposome formulation of MA consisting of a mixture of conventional and long-circulating (PEGylated) liposomes (LCP), with expected broader distribution among affected tissues of the mononuclear phagocyte system.
KEYWORDS: PEG, allopurinol, antimony, dogs, drug delivery, drug resistance, liposomes, meglumine antimoniate, visceral leishmaniasis
ABSTRACT
The treatment of dogs naturally infected with Leishmania infantum using meglumine antimoniate (MA) encapsulated in conventional liposomes (LC) in association with allopurinol has been previously reported to promote a marked reduction in the parasite burden in the main infection sites. Here, a new assay in naturally infected dogs was performed using a novel liposome formulation of MA consisting of a mixture of conventional and long-circulating (PEGylated) liposomes (LCP), with expected broader distribution among affected tissues of the mononuclear phagocyte system. Experimental groups of naturally infected dogs were as follows: LCP plus Allop, receiving LCP intravenously as 2 cycles of 6 doses (6.5 mg Sb/kg of body weight/dose) at 4-day intervals plus allopurinol at 30 mg/kg/12 h per os (p.o.) during 130 days (LCP+Allop); LC plus Allop, receiving LC intravenously as 2 cycles of 6 doses (6.5 mg Sb/kg/dose) plus allopurinol during 130 days (LC+Allop); Allop, treated with allopurinol only; and a nontreated control. Parasite loads were evaluated by quantitative PCR in liver, spleen, and bone marrow tissue and by immunohistochemistry in the ear skin, before treatment, just after treatment, and 4 months later. The LCP+Allop and LC+Allop groups, but not the Allop group, showed significant suppression of the parasites in the liver, spleen, and bone marrow 4 months after treatment compared to the pretreatment period or the control group. Only LCP+Allop group showed significantly lower parasite burden in the skin in comparison to the control group. On the basis of clinical staging and parasitological evaluations, the LCP formulation exhibited a more favorable therapeutic profile than the LC one, being therefore promising for the treatment of canine visceral leishmaniasis.
INTRODUCTION
Visceral leishmaniasis (VL) is a neglected disease caused by obligatory intracellular protozoa of the Leishmania donovani complex. It is fatal if left untreated in over 95% of cases. An estimated 50,000 to 90,000 new cases of VL occur worldwide each year. Most cases occur in Brazil, East Africa, and Southeast Asia. The parasite is transmitted to humans and dogs through the bites of infected female sand flies of the genera Lutzomyia and Phlebotomus in the New World and Old World, respectively. Depending on the etiological agent, the disease presents the following two distinct forms: anthroponotic VL that is endemic in India and East Africa, caused by L. donovani, and zoonotic VL that occurs in countries of the Mediterranean basin, Central Asia, and the Americas, caused by Leishmania infantum (World Health Organization website, https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis).
Domestic dogs are the most important urban reservoirs of L. infantum. The disease in dogs is characterized by a marked pleomorphism, and the clinical signs vary according to the immune response of the animals toward the infection. In general, the main clinical signs of canine visceral leishmaniasis (CVL) are dermopathy, lymphadenopathy, onychogryphosis, progressive weight loss, hematopoietic disorders, splenomegaly, polyuria and polydipsia, vomiting and diarrhea, and lesions originating from immune complex deposition in tissues (1). The treatment of dogs affected with VL uses mainly meglumine antimoniate (MA) or miltefosine, often in association with the leishmanistatic oral drug allopurinol (2, 3). However, despite the clinical recovery of most dogs during therapy, none of these drugs leads to a complete elimination of the parasite, and infected dogs must be kept under treatment for life (4). Thus, there is a great need of new more effective drugs to treat CVL.
The delivery of antimonial drugs using conventional liposomes (typically made from phosphatidylcholine and cholesterol) was found to improve up to 700-fold the drug activity in experimental VL (5). Previous studies performed in dogs with VL (6, 7) led to the idea that conventional liposomes containing an antimonial drug, although effective in reducing the parasite load and the parasite transmission to the vector, may not be sufficient alone to promote parasitological cure, probably because of the low drug targeting to less-accessible infection sites, such as the bone marrow or the skin. Thus, only when liposomal MA was given in combination with the leishmanistatic drug allopurinol was parasitological elimination in most tissues of naturally infected dogs observed in some of the treated animals (7).
As an attempt to further improve the efficacy of liposomal MA in CVL, PEGylation of MA-entrapped liposomes was investigated as a means of prolonging the vesicle blood circulation time and delivering MA to less-accessible mononuclear phagocyte system (MPS) tissues, including the bone marrow and the skin. The blood levels of MA were more prolonged from PEGylated liposomes than from conventional liposomes in both mice and dogs (8). An improved targeting of Sb to the bone marrow was also evidenced as a result of PEGylation. It has been proposed that MA-loaded conventional and PEGylated liposomes may act synergistically in the treatment of VL. The formulation consisting in a mixture of conventional and PEGylated liposomes was further evaluated in mice infected with L. infantum. It induced parasite suppression to a higher extent in both the spleen and bone marrow than did a PEGylated or conventional formulation alone. A recent evaluation of splenic CD4+ and CD8+ T cells in L. infantum-infected mice following treatment with the mixed-liposome formulation also supported the contribution of a Th1 immunoprotective response to the therapeutic efficacy (9).
This work reports for the first time, in dogs naturally infected with L. infantum, the therapeutic efficacy of the liposome formulation of MA consisting of a mixture of conventional and long-circulating (PEGylated) liposomes, in association with allopurinol. On the basis of clinical staging and parasitological evaluations, the novel formulation exhibited a more favorable therapeutic profile than that of the conventional liposomal formulation. Surprisingly, the Leishmania isolates from the bone marrow of dogs showed a much lower sensitivity to allopurinol than did those of a reference Leishmania strain.
RESULTS
Characterization of liposome formulations.
The formulations of MA in conventional and PEGylated liposomes showed, respectively, drug encapsulation efficiencies of 21.2% ± 0.4% and 29.8% ± 0.3%, vesicle hydrodynamic diameters of 159 ± 31 nm and 198 ± 27 nm, polydispersity indexes of 0.15 ± 0.04 and 0.23 ± 0.08, and zeta potentials of −24.0 ± 3.5 mV and −4.9 ± 0.7 mV. When the two formulations were mixed at equimolar lipid ratio, the suspension showed a mean hydrodynamic diameter of 209 ± 10 nm, polydispersity index of 0.26 ± 0.02, and zeta potential of −11.8 ± 1.8 mV.
Clinical and toxicity evaluations of dogs before and after treatment.
Treatment of mongrel dogs naturally infected with L. infantum was performed using two cycles of six doses of the conventional or the mixed-liposome formulation of MA (6.5 mg Sb/kg of body weight/dose) given intravenously (i.v.) at 4-day intervals, plus allopurinol (30 mg/kg/12 h per os) for 140 days starting 1 month before the first dose of the liposome formulation. Groups receiving only allopurinol and with no liposome treatment were used as controls.
These evaluations were performed just before and after treatment, as well as 4 months after the end of treatment, and consisted of the following exams: (i) physical examinations, (ii) a complete hemogram, (iii) a biochemical assessment of renal and liver functions, and (iv) a serological immunofluorescent antibody test (IFAT) and enzyme-linked immunosorbent assay (ELISA).
Before treatment, all animals presented clinical signs of CVL. Dermatological alterations were the main clinical signs observed (97.3% of the animals) and included onychogryphosis, hyperkeratosis of the muzzle, exfoliative dermatitis, seborrheic dermatitis, localized alopecia, and ulcerative dermatitis over joint prominences and the muzzle. The other most frequent clinical sign was lymphadenomegaly, observed in 89.2% of the dogs, with 54.5% of the animals with concomitant increase in submandibular, prescapular, and popliteal lymph nodes. Ophthalmopathies were recorded in 18.9% of the animals, most frequently being conjunctivitis/keratoconjunctivitis. Ultrasonography examinations also evidenced hepatomegaly and splenomegaly in 29.7% and 64.9% of the dogs, respectively. The hemogram showed anemia (43.2%), thrombocytopenia (37.8%), leukocytosis and lymphocytosis (78.4%), monocytosis (62.2%), and granulocytosis (40.5%). Although the serum biochemical profile showed no alteration in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels, hyperproteinemia was registered in 29.7% of the animals, and 64.8% of the dogs exhibited an albumin/globulin ratio below the reference value. All of the animals were also positive according to IFAT, with anti-Leishmania antibody titers ranging from 1:160 to 1:10,240.
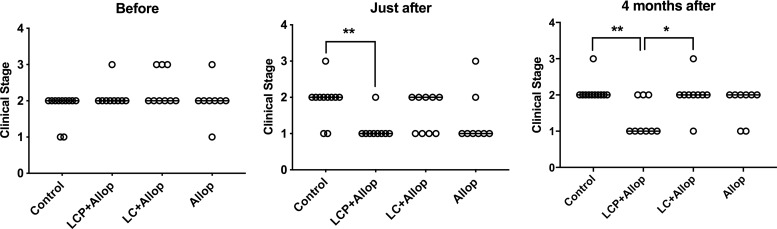
Taking into account clinical, laboratory, and serological data and a recently proposed clinical classification of CVL (7), each animal received a specific score, with the highest score corresponding to the most severe pattern of the disease. The distribution of animals was as follows: 8.1% of the dogs were in stage I, 78.4% were in stage II, and 13.5% were in stage III. This classification was also determined in each group just after and 4 months after treatment in order to evaluate the treatment efficacy from a clinical point of view.
Figure 1 compares this predictive clinical stage parameter between the different experimental groups. The data indicate that only the group with mixed-liposome formulation plus allopurinol (LCP+Allop) showed a significantly lower clinical score (i.e., severity of stages) than that of the control just after treatment and 4 months after treatment. Furthermore, after 4 months, a significantly higher proportion of animals with a low clinical score (=1) was encountered in the LCP+Allop group than that in the conventional liposome plus allopurinol (LC+Allop) group (P < 0.05, Fisher’s exact test).
FIG 1.
Clinical staging of L. infantum-infected dogs before and after treatment. Animals were treated with a mixed-liposome or conventional liposome formulation of MA plus allopurinol or allopurinol alone, and comparisons were made with nontreated animals (control). Liposome formulations (LCP, mixed-liposome formulation; LC, conventional liposome formulation) were given intravenously as two cycles of six doses (6.5 mg Sb/kg/dose) at 4-day intervals. Allopurinol (Allop) was given at 30 mg/kg/12 h per os for 130 days, starting 30 days before the first dose of liposomal MA. Animals remained without intervention during the 4 months after the treatment. The clinical scores are defined in details in reference 7. Score 0, absence of clinical signs and clinicopathological alterations of CVL and negative serology; score 1, mild clinical stage; score 2, moderate stage; score 3, severe stage; score 4, extremely severe disease stage. Data are shown as dot plots, and lines correspond to the medians (n = 8 to 11). *, P < 0.05, and **, P < 0.01, according to Fisher’s exact test, for a comparison of the proportion of dogs with a score of 1.
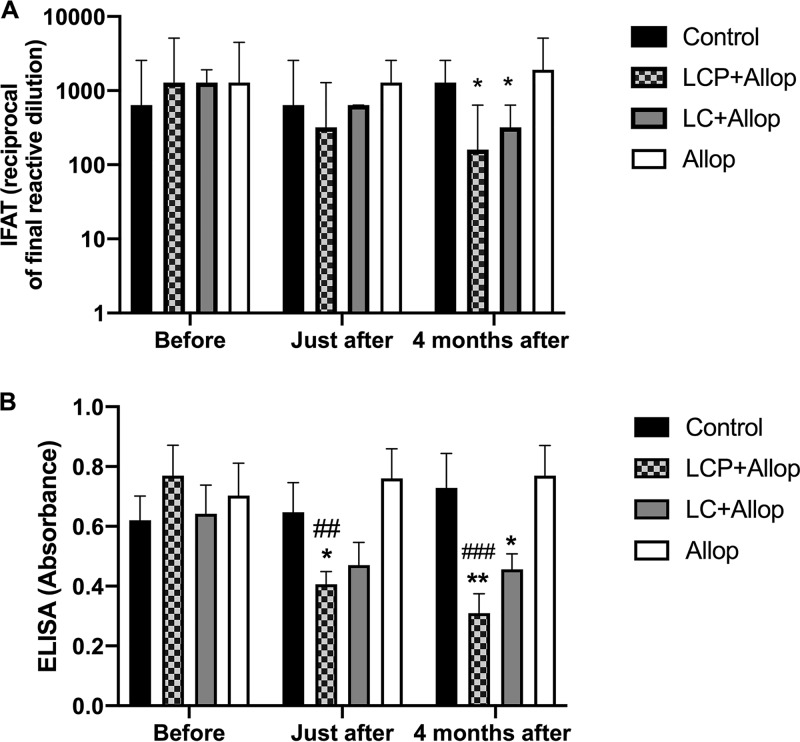
A low level of anti-Leishmania antibodies usually correlates with a more favorable prognosis (7). Figure 2 displays the evolution of antibody levels in the different groups as determined by IFAT (Fig. 2A) and ELISA (Fig. 2B). Interestingly, the LCP+Allop and LC+Allop groups exhibited significantly lower antibody levels than did the Allop group. LCP+Allop treatment also promoted a significant reduction in antibody level in comparison with the pretreatment period.
FIG 2.
Levels of anti-Leishmania antibodies in L. infantum-infected dogs before and after treatment. Animals were treated with a mixed-liposome or conventional liposome formulation of MA plus allopurinol or allopurinol alone, and a comparison was made with nontreated animals (control). (A) Assayed by IFAT. (B) Assayed by ELISA. Liposome formulations (LCP, mixed-liposome formulation; LC, conventional liposome formulation) were given intravenously as two cycles of six doses (6.5 mg Sb/kg/dose) at 4-day intervals. Allopurinol was given at 30 mg/kg/12 h per os for 130 days, starting 30 days before the first dose of liposomal MA. Animals remained without intervention during the 4 months after the treatment. (A) Data are shown as medians ± interquartile ranges; *, P < 0.05, according to Kruskal-Wallis test followed by Dunn’s multiple-comparison test, for comparison with the Allop group. (B) Data are shown as means ± standard error of the mean (SEM); *, P < 0.05, and **, P < 0.01 according to two-way ANOVA, followed by Tukey’s multiple-comparison test, for comparison with the Allop group; ##, P < 0.01, and ###, P < 0.001, according to two-way ANOVA, followed by Dunnett’s multiple-comparison test, for comparison with the pretreatment period.
The safety evaluations of the treatments were focused on markers of hepatic and renal functions (AST, ALT, urea, and creatinine levels) that were assessed and compared before and after treatment. The levels were within the reference range and showed no significant influence from the treatments (data not shown). Evaluation of a complete hemogram also showed no change as a function of time (data not shown).
On the other hand, transitory adverse reactions were observed during or shortly after bolus intravenous administration of the liposome formulations. In the LC+Allop group, the main symptoms were defecation, vomiting, and ataxia. In the LCP+Allop, group, those were defecation, sialorrhea, and vomiting. The percentage of animals in the LC+Allop group that presented symptoms in the first cycle of applications was 44%, and in the second cycle, all of them exhibited adverse effects. In the LCP+Allop group, in both cycles of application, 100% of the animals presented these side effects, and the occurrence of these effects was 76% higher than that in the LC+Allop group. Interestingly, during the first cycle of treatment with the mixed-liposome formulation, 33% of the dogs showed symptoms after the first dose, but the number of animals affected and the frequency of occurrence of adverse effects increased dramatically from the third dose. Despite the high occurrence rate of these effects in the dogs treated with the liposome formulations, all animals presented spontaneous resolution of symptoms between 1 and 5 min after the beginning of each observed reaction.
Parasitological evaluations in the liver, spleen, and bone marrow.
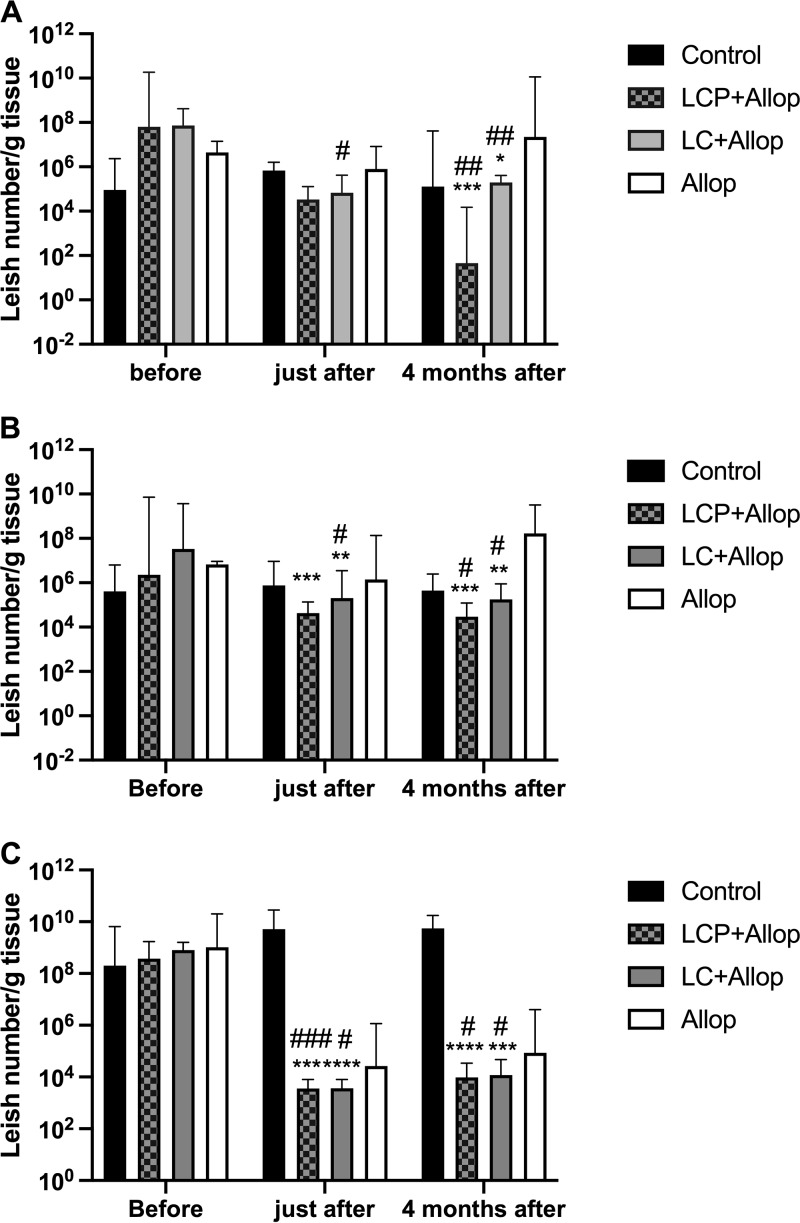
The therapeutic efficacy of the mixed-liposome formulation of MA plus allopurinol was also evaluated by quantitative PCR (qPCR) and compared to the efficacies of the conventional liposome formulation of MA plus allopurinol and of allopurinol alone. A comparison was also made with the nontreated group. Figure 3 shows the parasite burdens in the liver, spleen, and bone marrow of dogs naturally infected with L. infantum before and at different times after treatment.
FIG 3.
(A to C) Parasite burdens in the liver (A), spleen (B), and bone marrow (C) of L. infantum-infected dogs before and at different times after treatment. Animals were treated with a mixed-liposome or conventional liposome formulation of MA plus allopurinol or allopurinol alone, and a comparison was made with nontreated animals (control). Liposome formulations (LCP, mixed-liposome formulation; LC, conventional liposome formulation) were given intravenously as two cycles of six doses (6.5 mg Sb/kg/dose) at 4-day intervals. Allopurinol was given at 30 mg/kg/12 h per os for 130 days, starting 30 days before the first dose of liposomal MA. Animals remained without intervention during the 4 months after the treatment. Parasite burden was determined by qPCR. Data are shown as medians ± interquartile ranges; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, according to Kruskal-Wallis test, followed by Dunn’s multiple-comparison test, for comparison with Allop group (A and B) or control (C); #, P < 0.05; ##, P < 0.01; ###, P < 0.001, according to the Friedman test, followed by Dunn’s multiple-comparison test, for comparison with the pretreatment period. Leish, Leishmania.
In the liver and spleen (Fig. 3A and B), both liposome formulations promoted significant parasite suppressions 4 months after the treatment compared to the pretreatment period. The resulting parasite loads were also lower than those in the Allop group. Neither the Allop nor the control group showed significant changes in the parasite load. In the group that received the mixed-liposome formulation, 7 out of 9 animals had no detectable parasite in the liver 4 months after treatment (versus 3 out of 9 after the conventional liposome formulation). The levels of parasite suppression after liposome treatment were about the same in the spleen just after and 4 months after treatment, but they were less pronounced in the liver just after treatment than 4 months, suggesting a long-lasting action, especially in the case of the mixed formulation.
In the bone marrow (Fig. 3C), both liposome formulations promoted marked parasite suppressions just after (>5-log parasite reduction) and 4 months (>4-log parasite reduction) after the treatment compared to those in the pretreatment period and also to nontreated animals. Although the Allop group showed a reduction in the parasite burden, the change was not significant in comparison either to the pretreatment period or the control group.
Parasitological evaluations in the skin.
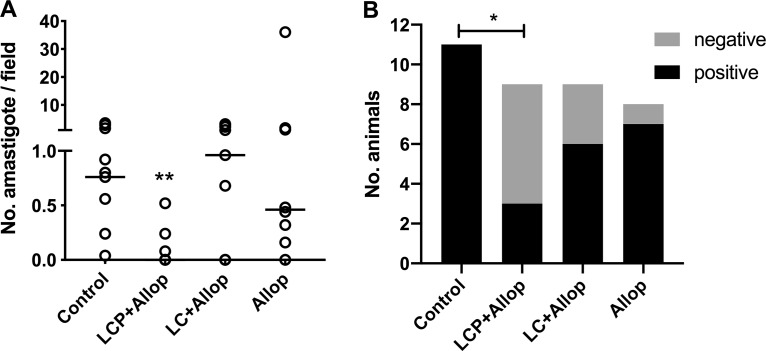
According to immunohistochemical evaluation of ear skin performed 4 months after treatment, only LCP+Allop group displayed significant parasite suppression in comparison to the control group (Fig. 4A). Moreover, only the LCP+Allop group showed a significantly lower proportion of positive dogs in comparison with control group (Fig. 4B).
FIG 4.
Parasite burden in the ear skin of L. infantum-infected dogs 4 months after treatment. Animals were treated with a mixed-liposome or conventional liposome formulation of MA plus allopurinol or allopurinol alone, and a comparison was made with nontreated animals (control). (A) Parasite burden determined by immunohistochemistry 4 months after the end of treatment. (B) Number of animals that were positive and negative according to immunohistochemistry in each experimental group. Liposome formulations (LCP, mixed-liposome formulation; LC, conventional liposome formulation) were given intravenously as two cycles of six doses (6.5 mg Sb/kg/dose) at 4-day intervals. Allopurinol was given at 30 mg/kg/12 h per os for 130 days, starting 30 days before the first dose of liposomal MA. Animals remained without intervention during the 4 months after the treatment. (A) Data are shown as aligned dot plot and medians. **, P < 0.01, according to a Kruskal-Wallis test, followed by Dunn’s multiple-comparison test, for comparison with the control. (B) *, P < 0.05, according to Fisher’s exact test for comparison of the proportion of negative dogs between the treated group and control.
Drug sensitivity of bone marrow Leishmania isolates.
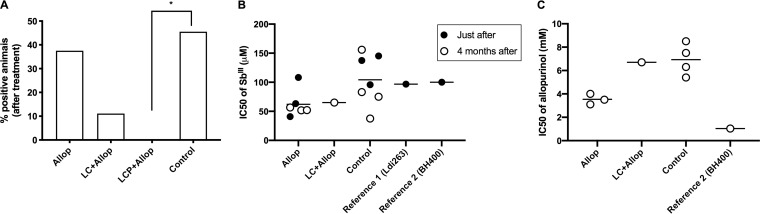
Bone marrow aspirate samples were obtained in dogs after treatment as an attempt to obtain Leishmania isolates for further evaluation of their sensitivities to Sb(III) and allopurinol. As shown in Fig. 5A, L. infantum isolates were obtained from dogs of the control, Allop, and LC+Allop groups but not from the LCP+Allop group. In the LC+Allop group, only one dog was positive regarding parasite isolation.
FIG 5.
(A to C) Leishmania parasite isolation from the bone marrow of dogs after different treatments (A) and sensitivity of the isolates to Sb(III) (B) and allopurinol (C) in comparison to reference Leishmania strains (Ldi263 and BH400). Dogs naturally infected with L. infantum were treated with a mixed-liposome or conventional liposome formulation of MA plus allopurinol or allopurinol alone or remained without treatment (control). Liposome formulations (LCP, mixed-liposome formulation; LC, conventional liposome formulation) were given intravenously as two cycles of six doses (6.5 mg Sb/kg/dose) at 4-day intervals Allopurinol was given at 30 mg/kg/12 h per os for 130 days, starting 30 days before the first dose of liposomal MA. Animals remained without intervention during the 4 months after the treatment. (A) Percentage of positive dogs regarding parasite isolation from the bone marrow; *, P < 0.05, according to Fisher’s exact test. (B) IC50s of Sb(III) of the isolates obtained in each animal group just after or 4 months after treatment. (C) IC50s of allopurinol of the isolates obtained in each animal group 4 months after the treatment.
Evaluation of the sensitivity to Sb(III) of the different Leishmania isolates showed 50% inhibitory concentration (IC50) values in the range of 35 to 155 μM, just like with reference L. infantum strains (Ldi263 and BH400), which had IC50s around 100 μM (Fig. 5B). The Leishmania isolate obtained from the LC+Allop group exhibited an intermediate IC50 of 65 μM. Thus, no evidence of treatment-induced resistance to Sb(III) was obtained.
Evaluation of the sensitivity to allopurinol of the different strains showed IC50 values in the range of 3 to 9 mM, which were much higher that of a reference strain (BH400) isolated from naturally infected dogs 16 years ago (Fig. 5C).
DISCUSSION
This work presents a new attempt to improve the efficacy of liposomal MA in CVL. The main differences with respect to the liposome formulation employed in our previous work (7) are the use of vesicles with diameter of 200 nm instead of 350 nm and the investigation of a mixture of conventional and PEGylated liposomes encapsulating MA, compared to a formulation of conventional liposomes. These new formulations were evaluated in combination with allopurinol for their therapeutic efficacies and toxicities in infected dogs, according to a new therapeutic protocol consisting of two 6-dose cycles with liposomal formulations, rather than just one cycle in the previous study, and using allopurinol at 30 mg/kg/12 h instead of 20 mg/kg/24 h. Furthermore, in the new regimen, animals remained untreated for 4 months before euthanasia to uncover possible relapses.
Here, the superiority of the mixed-liposome formulation, in comparison to the conventional one, is clearly established from both clinical and parasitological points of view. Four months after treatment, animals receiving the mixed formulation exhibited significantly better clinical outcome than did those receiving the conventional one. Although the two liposome formulations showed comparable efficacies regarding the level of suppression of parasites in the liver, spleen, and bone marrow, only the mixed-liposome formulation reduced significantly the skin parasite burden 4 months after treatment in comparison to the control group. The greatest effectiveness of the mixed-liposome formulation may be attributed to the long-circulating property of PEGylated liposomes in blood that may have contributed to vesicle extravasation through the leaky capillaries and drug accumulation in the skin infection sites, as a result of local inflammation (10). The reduction in parasite load in the skin is crucial, as this should result in the reduction or even blockade of parasite transmission to the sand fly. The long-term parasite suppression after treatment also represents an important achievement. Another question we intended to address in this study was whether the treatment may select drug-resistant parasites. However, the difficulty was isolating parasites from treated animals, especially those receiving the most effective drug. We were successful in isolating parasites only from the group treated with the conventional liposome formulation. In this case, no evidence of lower parasite sensitivity to Sb in the treated group was observed. Although encouraging, this result still requires confirmation with a larger number of isolates.
Importantly, the levels of markers of hepatic and renal functions (AST, ALT, urea, and creatinine) suffered no significant influence from the treatments and remained within the respective reference ranges. This indicates no major toxic effect of liposome accumulation in the liver. Kidney examination after euthanasia revealed that about 50% of the dogs that received allopurinol presented with xanthine urolithiasis (data not shown). This effect is frequently observed in dogs submitted to prolonged treatment with allopurinol (11), but the relatively high frequency reported here is probably due to the high allopurinol dosage used (12, 13). Nevertheless, no sign of renal dysfunction was observed.
The only adverse effects observed due to the liposome formulations were transitory hypersensitivity reactions, which were more pronounced after treatment with the mixed-liposome formulation than after conventional liposomes. The hypersensitivity reactions after bolus intravenous administration of conventional liposomes are in agreement with our previous studies (6, 7, 14). Those are attributed to the activation of the complement system by the lipid vesicles in a phenomenon known as complement activation-related pseudoallergy (CARPA) (15). Furthermore, PEGylated liposomes have also been reported to induce a hypersensitivity reaction from the second dose of administration through anti-polyethylene glycol (anti-PEG) IgM-mediated complement activation (16). These adverse reactions have been described for all of the liposomal drug formulations in clinical use. It is noteworthy that these effects can be avoided, or at least minimized, through premedication with antihistamines and steroids and a slow-infusion protocol (17).
In addition to the hypersensitivity reaction, the induction of anti-PEG IgM production and complement activation during the repeated administration of PEGylated liposomes is expected to increase their clearance from the blood circulation and enhance their accumulation in the liver through a phenomenon known as accelerated blood clearance (ABC) (16). This may explain the tendency toward higher efficacy and long-term action of the mixed-liposome formulation regarding parasite suppression in the liver.
The therapeutic efficacies of the liposomal formulations displayed here can be further compared to that of a conventional liposome formulation tested previously in naturally infected dogs (7). However, for adequate comparison, some differences between the actual and previous studies should be considered. First, naturally infected dogs were obtained from distinct regions of endemicity in Brazil (the Governador Valadares region in the present study versus the Ribeirão das Neves region in the previous one) and at different time periods (2006 versus 2016). This may result in L. infantum strains with distinct drug sensitivities. Second, the qPCR method employed here used a primer for kinetoplast DNA (kDNA) minicircles which makes the technique much more sensitive than that used previously with a primer for Leishmania DNA polymerase (18). Thus, a tissue sample found to be positive for Leishmania spp. in the present study may have appeared to be negative in the previous work. Third, although we used the same therapeutic agents, different treatment regimens were investigated.
Regarding the efficacy of the treatments, three important points can be highlighted. As a first point, treatment with allopurinol was found to be much less efficacious in the present study, despite the higher dose used. This may be due to a lower drug sensibility of the Leishmania strains infecting these dogs. This assumption is supported by the observation that the isolated strains were much less sensitive to allopurinol than was a strain isolated from dogs 16 years ago. It is possible that the extensive use of allopurinol as chemotherapeutic agent for CVL has resulted in the selection of drug-resistant or at least more-tolerant Leishmania strains. This hypothesis is consistent with the recent report of the association of allopurinol resistance in L. infantum with disease relapse in the canine host (19). Second, the conventional liposome formulation tested here is less effective than that evaluated previously, despite the higher total dose of Sb used. This discrepancy may arise from differences in the liposome characteristics or parasite drug sensitivities. The liposome formulation used here displays a higher lipid/Sb ratio and smaller vesicle size, resulting in a smaller amount of Sb per vesicle and, presumably, slower drug accumulation in the liver and spleen (8). On the other hand, the lower effectiveness of allopurinol may also compromise the synergistic actions between Sb and allopurinol. Third, while marked parasite clearance was observed in the liver, mainly after the mixed-liposome formulation, parasites were detected in the bone marrow aspirate samples of all dogs. This is in contrast with the previous study in which 50% of dogs were found to have no parasite in the bone marrow. Possible explanations are the higher sensitivity of the qPCR method used here or the possibly weaker synergistic actions between Sb and allopurinol. Thus, our data suggest that, although very encouraging, the therapeutic efficacy of the mixed-liposome formulation of MA could be further improved through combination with an antileishmanial agent other than allopurinol. Possible candidates to substitute allopurinol comprise immunomodulatory agents.
As a major contribution, the present work provides the proof of concept in naturally infected dogs that the effectiveness of a formulation made from a mixture of conventional and PEGylated liposomes is greater than that made from conventional liposomes, supporting the therapeutic benefit of broader liposome distribution and drug delivery among the infected tissues. Although only an antimonial drug was used in the present study, this concept can possibly be extended to other drugs in clinical use, such as miltefosine or amphotericin B.
Another important issue to be discussed here is the potential of the mixed-liposome formulation to become a clinical candidate. The fact that no liposomal drug product has reached the market since the first description of efficacy of liposome-encapsulated antimonial in dogs, about 40 years ago (20), highlights a slow development, most probably due technological challenges related to the large-scale production and long-term stability of liposomal drug formulations. On the other hand, significant progress regarding these aspects has been achieved during the period, through the development of liposomal formulations of anticancer and antifungal agents now available on the market for human use (21). In the same direction, our group also markedly improved the processes used to prepare liposomal formulations of MA (5, 9). The method used here is based on the freeze-drying of empty liposomes in the presence of cryoprotective sugar and the subsequent rehydration of the lyophilized liposomes with an aqueous solution of the antimonial drug. Using this scheme, the liposomal formulation may be produced as preformed freeze-dried empty vesicles, and rehydration may be performed just before administration. This circumvents the stability problems arising from the long-term storage of liposomes in the presence of water-soluble drugs. Thus, the process employed in our study, owing to its simplicity, scalability, and reduced number of steps, appears to be more suitable than those previously described for the large-scale production of such liposome formulations (5). The proposal of mixed-liposome formulation may be claimed to increase the complexity of production since it involves the preparation of two different liposome suspensions. Nevertheless, this process may be further simplified by first preparing conventional liposomes and then submitting part of this suspension to postinsertion of PEG phospholipid, as described recently (22). Thus, it is the opinion of the authors in the current study that the emergence of liposomal drug formulation for CVL is just a matter of time. In comparison to the treatment currently available for CVL involving either MA or miltefosine, the use of a mixed-liposome formulation may present the following advantages: (i) lower frequency of dosing, (ii) reduced side effects, (iii) greater efficacy, and (iv) lower risk of induction of drug resistance.
Even though the transmission of drug-resistant Leishmania strains from treated dogs to humans has not yet been established in the field, the public health authorities of most affected countries do not allow in dogs the use of drugs already used in humans. This means that a liposomal formulation of MA would probably not be allowed for CVL in countries that use pentavalent antimonials as first-line drugs in humans. Thus, the new formulations of MA may be used to treat dogs in Europe, where antimonials are not used any more, but not in Brazil.
Alternatively, our formulation may find application in the treatment of human leishmaniases. Indeed, due to its broad distribution among MPS tissues, the mixed formulation is promising for the treatment of disseminated forms of leishmaniasis, like Post-kala-azar dermal leishmaniasis, HIV-VL coinfection, and disseminated cutaneous and mucocutaneous leishmaniases.
In conclusion, this study establishes for the first time, in dogs naturally infected with L. infantum, the higher therapeutic efficacy of a mixed formulation of conventional and PEGylated liposomes than that with a conventional liposome formulation. The data also suggest a long-lasting action of this formulation in the skin and liver.
MATERIALS AND METHODS
Materials and drugs.
Cholesterol and dicetyl phosphate were purchased from Sigma Co. (St. Louis, MO). Distearoylphosphatidylcholine (DSPC) and distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG) were obtained from Lipoid (Ludwigshafen, Germany). N-Methyl-d-glucamine and antimony pentachloride (SbCl5, 99%) were obtained from Aldrich Chemical Co. (Milwaukee, WI, USA). Allopurinol was purchased from a commercial laboratory for pharmaceutical products (FarmaVet, Brazil) as oral capsules at individual dosages of 30 mg/kg of body weight. The content of allopurinol was certified as >98% of the informed value by the Center for Studies and Analitical Pharmaceutical Development (CEDAFAR) of the Universidade Federal de Minas Gerais (UFMG).
Meglumine antimoniate (MA) was synthesized, as previously described (23), from equimolar amounts of N-methyl-d-glucamine and pentavalent antimony oxyhydrate freshly prepared from the hydrolysis of antimony pentachloride in water. The resulting product contained 28% Sb by weight. As previously established (24), synthetic MA and commercial MA (Glucantime; Sanofi-Aventis Farmacêutica Ltd., São Paulo, Brazil) are interchangeable in the preparation of the liposomal formulation.
Animals.
The present study was approved by the Ethical Committee for Animal Use of Nanocore (protocol number 01/2014), and all procedures were carried out according to the international guidelines. This research was registered in the SisGen platform as A087E97, due to access to Brazilian genetic heritage.
Thirty-seven mongrel dogs (17 males and 20 females) weighing 9.8 ± 4 kg (mean ± standard deviation) of unknown age (but older than 1 year) and naturally infected with L. infantum were obtained by donation from the Center for Control of Zoonosis (CCZ) of Governador Valadares City (Minas Gerais State, Brazil), an area where canine visceral leishmaniasis is endemic. These infected dogs were initially identified by serological tests (Dual-Path Platform [DPP] and ELISA) performed by the CCZ and captured as part of the activities of the municipality’s VL Control Program.
The serological diagnosis was confirmed at the Laboratório de Bioensaios em Leishmania (Department of Parasitology/ICBIM), Federal University of Uberlândia (UFU), using an indirect immunofluorescence assay (IFAT) and ELISA (25). All animals were found to be positive by IFAT (≥1:40 serum dilution) and ELISA (optical density, >0.1 at a 1:400 serum dilution). In addition, specific PCR of bone marrow aspirate samples was used to confirm the infection of the animals by L. infantum (26).
The animals were kept in the experimental kennel at Nanocore Biotecnologia S.A. (Esmeralda, Minas Gerais, Brazil) with drinking water and balanced commercial food (Quatree Gourmet Cães Adultos, Granvita, Brazil) ad libitum during the entire experimental period. Prior to treatment, dogs were treated against intestinal helminths (Vermivet Plus; Biovet, Brazil) and ectoparasite infestations (Frontline Top Spot; Merial, Brazil) and immunized against rabies (Defensor; Pfizer Saúde Animal, Brazil) and other infectious diseases (Vanguard HTLP 5/CV-L; Pfizer Saúde Animal).
The kennel consisted of collective bays, with dimensions of 5 by 2.5 m, each one receiving 5 animals or fewer. All bays were provided with covered areas and open areas (solarium) to allow adequate ventilation and sunlight. The whole kennel was, without exception, covered by nylon mesh screens suitable for preventing any possibility of access by sand flies (natural invertebrate host of L. infantum) to infected dogs, in order to promote the biosafety of the persons involved in the experiment and of the inhabitants and animals of the region. Before arrival of the animals, a sanitary void was performed, and all internal and external walls were sprayed with residual insecticide based on deltamethrin at 25 mg/m2 (K-Otrine CE25; Bayer CropScience Ltd., Brazil), including mosquito net screens.
Preparation and characterization of liposome formulations.
Two different liposome formulations incorporating MA were prepared, one consisting of conventional liposomes made from DSPC, CHOL, and DCP at a molar ratio of 5:4:1 (named LC), and the other one consisting of PEGylated liposomes made from DSPC, CHOL, DCP, and DSPE-PEG at molar ratio of 4.7:4:1:0.47.
The encapsulation of MA in liposomes was performed as described previously (9). Briefly, multilamellar liposomes were prepared in deionized water at a final lipid concentration of 55 g/liter. These multilamellar liposomes were transformed into unilamellar vesicles through five freeze-thaw cycles, followed by repeated extrusions (10 times) across 100-nm-pore-size polycarbonate membranes. The resulting liposome suspensions were mixed with an aqueous sucrose solution at a 3:1 sugar/lipid mass ratio. The mixtures were then immediately frozen in liquid nitrogen and subsequently freeze dried for 48 h (freeze-dryer L101; Liotop, São Carlos/São Paulo, Brazil). Rehydration of the dried powder was performed with a solution of MA in water (Sb concentration, 40 g/liter), as follows. A total of 100% of the original liposome volume of the MA solution was added to the lyophilized powder, and the mixture was vortexed and incubated for 45 min at 65°C. The liposome suspensions were then diluted 1:6 with phosphate-buffered saline (PBS; 0.15 M NaCl, 10 mM phosphate [pH 7.2]) and subjected to centrifugation (23,000 × g, 40 min, 15°C). The liposome pellet was finally resuspended in 0.15 M NaCl at a final lipid concentration of 67 g/liter.
To prepare the formulation consisting of a mixture of conventional and PEGylated liposomes (named LCP), both liposomal MA formulations were mixed at 25°C at an equimolar lipid ratio just before administration.
The mean hydrodynamic diameter, polydispersity index, and the zeta potential of the vesicles in suspension were determined by photon correlation spectroscopy at 25°C using a particle size analyzer (Zetasizer S90; Malvern, UK). The amount of Sb was determined in the resulting liposome suspension, after digestion of the sample with nitric acid, by electrothermal atomic absorption spectrometry (Analyst AA600; Perkin-Elmer, Inc., Waltham, MA, USA), as described previously (8).
Treatment protocols.
Dogs were randomly distributed into four groups of 8 to 11 animals, as follows. In the LCP+Allop group, nine animals were treated with two cycles of six intravenous doses of LCP (mixture of conventional and PEGylated liposomal MA formulations at an equimolar lipid ratio) at 6.5 mg Sb/kg/dose given at 4-day intervals, with an interval of 40 days between both cycles, and allopurinol at 30 mg/kg/12 h per os for 130 days starting 30 days before the first dose of LCP. In the LC+Allop group, nine animals were treated with two cycles of six intravenous doses of LC (conventional liposomal MA formulation) at 6.5 mg Sb/kg/dose given at 4-day intervals, with an interval of 40 days between both cycles, and allopurinol (30 mg/kg/12 h per os) for 130 days starting 30 days before the first dose of LC. In the Allop group, eight animals were treated with allopurinol at 30 mg/kg/12 h per os for 130 days. In the control group, 11 animals remained without treatment.
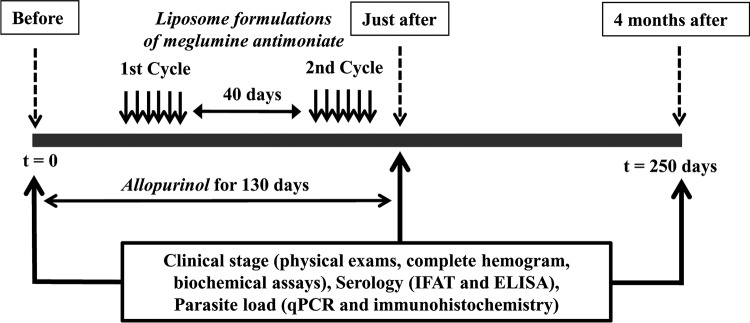
After the end of treatment (on day 130), all animals of the four experimental groups were kept in the kennel for 120 additional days without any intervention, representing a total experimental period of 250 days. Animals were clinically monitored during the experimental period and were submitted to clinical and parasitological evaluations just before and on days 130 and 250 after the beginning of treatment (Fig. 6).
FIG 6.
Scheme illustrating the treatment regimen of dogs naturally infected with L. infantum. Dogs were treated with mixed or conventional liposome formulation of MA plus allopurinol or allopurinol alone. Liposome formulations (LCP, mixed-liposome formulation; LC, conventional liposome formulation) were given intravenously as two cycles of six doses (6.5 mg Sb/kg/dose) at 4-day intervals. Allopurinol (Allop) was given at 30 mg/kg/12 h per os for 130 days, starting 30 days before the first dose of liposomal MA. The samples of tissues and blood were collected from all animals and clinically evaluated in times before, just after, and 4 months after treatment protocols. No intervention was performed on animals for 4 months after treatment for assessment of relapses.
At day 250, the animals were euthanized according to the recommendations of the Federal Council of Veterinary Medicine and the Guidelines for Euthanasia Practice (2013) of the National Council for Control of Animal Experimentation (CONCEA). First, a preanesthetic protocol was applied, consisting of 22 mg/kg ketamine hydrochloride (Ketamina Agener; Agener União, Brazil) and 2 mg/kg xylazine hydrochloride (Calmiun; Agener União) by the intravenous route. Then, an overdose of 60 mg/kg sodium thiopental (Thiopentax 1g; Cristália, Brazil) was performed by slow endovenous injection to induce cardiorespiratory arrest.
During the initial experimental period, 3 animals died, with one dog in the Allop group, one in the LC+Allop group, and one in the LCP+Allop group, most probably due to the natural evolution of the disease.
Clinical and toxicity evaluations.
Just before treatment and on days 130 and 250 after the start of treatment, the dogs were inspected for the presence of clinical signs of CVL and possible toxicity of the treatments. Therefore, serum and plasma (using EDTA as an anticoagulant) were collected for quantitative evaluation of anti-Leishmania antibodies (IFAT and ELISA), complete hemogram (erythrogram, leukogram, and total platelet count), and levels of serum urea, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin, total proteins, globulins, and albumin, and the albumin/globulin (A/G) ratio.
Based on the results of the physical examination, levels of anti-Leishmania antibodies determined by IFAT and ELISA (25), and laboratory findings in hemogram and serum biochemistry, the animals were classified according to a previously proposed staging system (7). Each animal received a score ranging from 0 to 4, where score 0 corresponds to absence of clinical signs of CVL, negative serology (IFAT, ≤ 1:40; ELISA with optical density of ≤0.1 at 1:400 serum dilution), and no abnormalities in hemogram and serum biochemistry, and score values from 1 to 4 correspond to the mild, moderate, severe, and extremely severe disease clinical stages, respectively.
As an attempt to uncover possible differences between the liposome formulations regarding the occurrence of complement-mediated pseudoallergic reactions (6), animals were monitored for clinical and behavioral changes during the injection and in the 5 h following administration.
Tissue sample collection.
In order to obtain samples of bone marrow, liver, spleen and skin before and just after treatment, dogs were submitted to general anesthesia using the combination of 2 mg/kg of body weight of xylazine hydrochloride and 11 mg/kg of body weight of ketamine hydrochloride by intravenous injection, followed by trichotomy and antisepsis of the site with a 2% iodine alcohol solution. Then, 1 ml of sternal bone marrow aspirate was collected, followed by 1 ml of spleen and liver aspirates after previous localization of the organs using portable ultrasound equipment (SonoHeart Elite Superior 180 Plus; SonoSite, Inc., USA). Skin fragments of the internal face of the right ear were collected with the aid of a 5-mm punch for sterile biopsy (Punch para Biópsia; Kolplast Ltd., Brazil). Bone marrow samples were also collected as described above just before euthanasia after general anesthesia. All other tissue samples obtained on day 250 were collected during the necropsy. The tissue samples to be used for parasite quantification by quantitative PCR (qPCR) were stored at –20°C, and by immunohistochemistry (IHC), the samples of ear skin were fixed in a 10% neutral-buffered formalin solution until required for further processing.
qPCR determination of parasite load in the liver, spleen, and bone marrow.
The parasite loads in the liver, spleen, and bone marrow of the dogs were determined by qPCR. First, extraction of the genomic DNA was carried out from 20 mg of each tissue sample using a commercial kit (ReliaPrep genomic DNA [gDNA] tissue miniprep system kit; Promega Co., USA), following the manufacturer’s instructions. The DNA concentration and 260 nm/280 nm ratio were determined using a spectrophotometer (NanoDrop Lite spectrophotometer; Thermo Fisher Scientific, USA), and the samples were stored at −20°C until further processing. The qPCRs were performed in triplicate in 96-well plates and processed on a thermocycler (StepOne real-time PCR system; Applied Biosystems, USA). In order to quantify parasite burden, specific primers [forward L150, 5′-GGG (G/T)AG GGG CGT TCT(G/C)CG AA-3′; and reverse L152, 5′-(G/C)(G/C)(G/C) (A/T)CT AT(A/T) TTA CAC CAACCC C-3′] were used to amplify a 120-bp fragment of the conserved region of kinetoplast DNA (kDNA) minicircles of Leishmania spp. (27). The reactions took place in a 25-μl final volume containing 100 nmol/liter each primer, 1× GoTaq qPCR master mix, and 50 ng of DNA template. The optimized qPCR conditions were as follows: an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min. At the end of the reactions, the temperature was elevated gradually until dissociation of double strands of the amplified material to check for possible contamination of the sample with DNA or dimers of the primers.
The number of L. infantum cells in each sample was determined from a standard curve constructed through serial dilutions of L. infantum promastigotes (strain MCAN/BR/2002/BH401) added to the corresponding tissue (20 mg) from noninfected dogs of a region of nonendemicity on a range of 100 to 106 parasites/mg of tissue. After DNA extraction, a standard curve was obtained by linear regression that correlated the known amount of L. infantum cells with the cycle threshold (CT) given by the amplification of each point of the curve. In the analysis of the results, only reactions with an efficiency close to 100% and a standard curve with good correlation coefficient (r2 = 0.970 to 0.999) were considered (28). The DNA integrity in the samples was also confirmed by conventional PCR (29) through amplification of a 307-bp fragment of the β-actin gene, constitutively expressed in all mammalian cells, using the following primers: forward, 5′-CTT CTA CAA CGA GCT GCG CG-3′; and reverse, 5′-TCA TGA GGT AGT CGG TCA GG-3′ (7, 28). The following quantification limits could be determined: liver, 0.046 Leishmania cells/mg; spleen, 0.12 Leishmania cells/mg; and bone marrow, 0.104 Leishmania cells/mg.
IHC and morphometrical methods for parasite load determination in the skin.
Just after euthanasia, samples of ear skin were collected and fixed in 10% neutral-buffered formalin solution. All tissue samples were dehydrated, cleared, embedded in paraffin, and cut into 4- to 5-μm-thick sections for immunohistochemical studies. Deparaffinated slides were hydrated and incubated with 4% (vol/vol) hydrogen peroxide in 0.01 M PBS (pH 7.2) to block endogenous peroxidase activity, followed by 30 min of incubation with powdered skim milk (diluted to 12 g/200 ml) to block nonspecific immunoglobulin absorption to tissues. A heterologous hyperimmune serum from dogs naturally infected with L. infantum (IFAT, ≥1:40), diluted 1:10 in 0.01 M PBS, was used as primary antibody “cross-reactive,” as described previously (30), with the following modifications. The slides were incubated from 18 to 22 h at 4°C in a humid chamber. After washing in PBS, the slides were incubated with biotinylated goat anti-mouse and anti-rabbit (LSAB2 kit, catalog no. KO675-1; Dako, Carpinteria, CA, USA), washed in PBS again, and then incubated with a streptavidin-peroxidase complex (LSAB2 kit, catalog no. K0675-1; Dako) for 30 min each at room temperature. The reaction was developed with a 0.024% diaminobenzidine (DAB) solution (Sigma, St. Louis, MO, USA) and 0.16% (vol/vol) hydrogen peroxide. Finally, slides were dehydrated, cleared, counterstained with Harris’ hematoxylin, and mounted with coverslips.
The images were obtained by light microscopy (BX40F-3; Olympus Optical Co. Ltd., Japan) with a camera attached (Q-Color 3; Olympus America, Inc., Canada), in a 40× objective, viewed on a computer screen, and relayed to an image analysis system. To determine the Leishmania tissue load, morphometric analysis was performed by counting the immunolabeled amastigotes in 25 randomly selected microscopic fields and analyzed using the ImageJ software (https://imagej.nih.gov/ij/).
Parasite isolation and evaluation of sensitivity to Sb and allopurinol.
About 1 ml of bone marrow aspirates was collected directly in 5 ml of minimum essential medium alpha (MEM α; Gibco, Invitrogen, NY) and then seeded in MEM α supplemented with 10% (vol/vol) inactivated fetal bovine serum (catalog no. 10270106; Gibco, Thermo Fisher), 100 μg/ml kanamycin, 50 μg/ml ampicillin, 2 mM l-glucamine, 100 nM folic acid, 82 nM d-biotin, 3 μM adenine, 1 μg/ml hemin, and 10 μM biopterin at pH 7.04 and incubated at 25°C. Every 10 days, all of the cultures were examined for the presence of promastigote forms of the parasite. Negative cultures were then supplemented with fresh medium and were reexamined after 10 days; if still negative, the operation was repeated until 2 months.
The following L. infantum isolates were obtained: MCAN/BR/2016/52(4), MCAN/BR/2016/57(4), MCAN/BR/2016/90(4), MCAN/BR/2016/121(4), MCAN/BR/2016/138(4), MCAN/BR/2016/140(4), MCAN/BR/2016/164(4), and MCAN/BR/2016/177(4). A comparison was made with the reference strains MCAN/BH/2000/BH400 (called BH400) and MHOM/MA/67/ITMAP-263 (called Ldi263).
The drug susceptibility of the Leishmania strains was determined through exposition of the promastigote cells to increasing concentrations of potassium antimonyl tartrate [Sb(III)] or allopurinol (FarmaVet, Brazil) and evaluation of the parasite growth through measurement of the turbidity at 600 nm using a Molecular Devices SpectraMax M5 microplate reader (31). Briefly, 1 × 106 log-phase promastigotes were seeded in 24-well cell culture plates with 1.5 ml of complete MEM α in the presence of drug at various concentrations. Nontreated parasites were established for growth comparison. Parasites were then incubated at 25°C for 72 h under shaking. The drug concentration that inhibits parasite growth by 50% in relation to nontreated cells (IC50) was calculated by based on concentration-response curves applying a sigmoidal dose-response equation with variable slope carried out using the software Prism version 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Statistical analysis.
Statistical analyses were performed with the aid of Prism version 8.00 for Windows (GraphPad Software, USA). According to the Kolmogorov-Smirnov test, the most experimental data were not normally distributed; only ELISA data were normally distributed. Then, the Kruskal-Wallis test followed by Dunn’s multiple-comparison test or the Friedman test followed by Dunn’s multiple-comparison test were used to compare levels of anti-Leishmania antibodies by IFAT and parasitic loads by qPCR in the liver, spleen, and bone marrow, with the parasitic load by IHC in the skin. The two-way analysis of variance (ANOVA), followed by Tukey’s or Dunnett’s multiple-comparison test, was used to compare levels of anti-Leishmania antibodies by ELISA. Fisher’s exact test was used to compare the clinical stage, the proportions of negative dogs between the treated group and control group, and the percentage of positive dogs regarding parasite detection in bone marrow and skin. A significance level of 95% was applied in all statistical tests.
Data availability.
Data related to this paper may be found at https://doi.org/10.6084/m9.figshare.11904954.
ACKNOWLEDGMENTS
We thank Nayara K. L. M. Moura, Adriel A. F. Ferreira, and employees of the experimental kennel of Nanocore Biotecnologia S.A. for technical support. We are also grateful to the Centro de Controle de Zoonoses de Governador Valadares, Minas Gerais, Brazil and the Centro de Referência em Leishmanioses, FIOCRUZ/Minas, Brazil, for performing rK39 and direct agglutination tests in the employees of Nanocore.
This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 305659/2017-0, 402634/2013-6, and studentship grants), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, grant APQ-03129-16), Pró-Reitoria de Pesquisa da UFMG (PRPq/UFMG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, studentship grant). F.F. and C.D. are recipients of a research fellowship from CNPq.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Solano-Gallego L, Koutinas A, Miro G, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G. 2009. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol 165:1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Canavate C, Molina R, Moreno J, Nieto J. 2004. Canine leishmaniasis. Adv Parasitol 57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro RR, Michalick MSM, da Silva ME, Dos Santos CCP, Frézard FJG, da Silva SM. 2018. Canine leishmaniasis: an overview of the current status and strategies for control. Biomed Res Int 2018:3296893. doi: 10.1155/2018/3296893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miró G, Cardoso L, Pennisi MG, Oliva G, Baneth G. 2008. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part two. Trends Parasitol 24:371–377. doi: 10.1016/j.pt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Frézard F, Demicheli C. 2010. New delivery strategies for the old pentavalent antimonial drugs. Expert Opin Drug Deliv 7:1343–1358. doi: 10.1517/17425247.2010.529897. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro RR, Moura EP, Pimentel VM, Sampaio WM, Silva SM, Schettini DA, Alves CF, Melo FA, Tafuri WL, Demicheli C, Melo MN, Frézard F, Michalick M. 2008. Reduced tissue parasitic load and infectivity to sand flies in dogs naturally infected by Leishmania (Leishmania) chagasi following treatment with a liposome formulation of meglumine antimoniate. Antimicrob Agents Chemother 52:2564–2572. doi: 10.1128/AAC.00223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva SM, Amorim IFG, Ribeiro RR, Azevedo EG, Demicheli C, Melo MN, Tafuri WL, Gontijo NF, Michalick MSM, Frézard F. 2012. Efficacy of combined therapy with liposome-encapsulated meglumine antimoniate and allopurinol in treatment of canine visceral leishmaniasis. Antimicrob Agents Chemother 56:2858–2867. doi: 10.1128/AAC.00208-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo EG, Ribeiro RR, da Silva SM, Ferreira CS, de Souza LE, Ferreira AA, de Oliveira e Castro RA, Demicheli C, Rezende SA, Frézard F. 2014. Mixed formulation of conventional and pegylated liposomes as a novel drug delivery strategy for improved treatment of visceral leishmaniasis. Expert Opin Drug Deliv 11:1551–1560. doi: 10.1517/17425247.2014.932347. [DOI] [PubMed] [Google Scholar]
- 9.Reis LES, Fortes De Brito RC, De Oliveira Cardoso JM, Mathias FAS, Aguiar Soares RDO, Carneiro CM, De Abreu Vieira PM, Ramos GS, Frézard FJG, Roatt BM, Reis AB. 2017. Mixed formulation of conventional and pegylated meglumine antimoniate-containing liposomes reduces inflammatory process and parasite burden in Leishmania infantum-infected BALB/c mice. Antimicrob Agents Chemother 61:e00962-17. doi: 10.1128/AAC.00962-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boerman OC, Oyen WJG, van Bloois L, Koenders EB, van der Meer JWM, Corstens FHM, Storm G. 1997. Optimization of technetium-99m-labeled PEG liposomes to image focal infection: effects of particle size and circulation time. J Nucl Med 38:489–493. [PubMed] [Google Scholar]
- 11.Torres M, Bardagí M, Roura X, Zanna G, Ravera I, Ferrer L. 2011. Long term follow-up of dogs diagnosed with leishmaniosis (clinical stage II) and treated with meglumine antimoniate and allopurinol. Vet J 188:346–351. doi: 10.1016/j.tvjl.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Noli C, Auxilia ST. 2005. Treatment of canine Old World visceral leishmaniasis: a systematic review. Vet Dermatol 16:213–232. doi: 10.1111/j.1365-3164.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 13.Reguera RM, Morán M, Pérez-Pertejo Y, García-Estrada C, Balaña-Fouce R. 2016. Current status on prevention and treatment of canine leishmaniasis. Vet Parasitol 227:98–114. doi: 10.1016/j.vetpar.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro RR, Moura EP, Sampaio WM, Silva SM, Fulgêncio GO, Tafuri WL, Michalick MSM, Frézard F. 2013. Complement activation-related pseudoallergy in dogs following intravenous administration of a liposomal formulation of meglumine antimoniate. Pesq Vet Bras 33:1016–1020. doi: 10.1590/S0100-736X2013000800012. [DOI] [Google Scholar]
- 15.Szebeni J. 2005. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology 216:106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Lila ASA, Kiwada H, Ishida T. 2013. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release 172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Szebeni J, Bedőcs P, Urbanics R, Bünger R, Rosivall L, Tóth M, Barenholz Y. 2012. Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: a porcine model. J Control Release 160:382–387. doi: 10.1016/j.jconrel.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G, The LeishVet Group . 2011. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasur-Landau D, Jaffe CL, David L, Baneth G. 2016. Allopurinol resistance in Leishmania infantum from dogs with disease relapse. PLoS Negl Trop Dis 10:e0004341. doi: 10.1371/journal.pntd.0004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman WL Jr, Hanson WL, Alving CR, Hendricks LD. 1984. Antileishmanial activity of liposome-encapsulated meglumine antimonate in the dog. Am J Vet Res 45:1028–1030. [PubMed] [Google Scholar]
- 21.Pattni BS, Chupin VV, Torchilin VP. 2015. New developments in liposomal drug delivery. Chem Rev 115:10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 22.Mare R, Paolino D, Celia C, Molinaro R, Fresta M, Cosco D. 2018. Post-insertion parameters of PEG-derivatives in phosphocholine-liposomes. Int J Pharm 552:414–421. doi: 10.1016/j.ijpharm.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Demicheli C, Ochoa R, Lula IS, Gozzo FC, Eberlin MN, Frézard F. 2003. Pentavalent organoantimonial derivatives: two simple and efficient synthetic methods for meglumine antimonate. Appl Organometal Chem 17:226–231. doi: 10.1002/aoc.425. [DOI] [Google Scholar]
- 24.Frézard F, Michalick MS, Soares CF, Demicheli C. 2000. Novel methods for the encapsulation of meglumine antimoniate into liposomes. Braz J Med Biol Res 33:841–846. doi: 10.1590/s0100-879x2000000700016. [DOI] [PubMed] [Google Scholar]
- 25.Amorim IFG, da Silva SM, Figueiredo MM, Moura EP, de Castro RS, Lima TKS, Gontijo NF, Michalick MSM, Gollob KJ, Tafuri WL. 2011. Toll receptors type-2 and CR3 expression of canine monocytes and its correlation with immunohistochemistry and xenodiagnosis in visceral leishmaniasis. PLoS One 6:e27679. doi: 10.1371/journal.pone.0027679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS. 2009. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol 166:159–162. doi: 10.1016/j.vetpar.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Degrave W, Fernandes O, Campbell D, Bozza M, Lopes U. 1994. Use of molecular probes and PCR for detection and typing of Leishmania–a mini-review. Mem Inst Oswaldo Cruz 89:463–469. doi: 10.1590/s0074-02761994000300032. [DOI] [PubMed] [Google Scholar]
- 28.Alves CF, de Amorim IF, Moura EP, Ribeiro RR, Alves CF, Michalick MS, Kalapothakis E, Bruna-Romero O, Tafuri WL, Teixeira MM, Melo MN. 2009. Expression of IFN-gamma, TNF-alpha, IL-10 and TGF-beta in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol 128:349–358. doi: 10.1016/j.vetimm.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 30.Tafuri WL, Santos RL, Arantes RM, Gonçalves R, de Melo MN, Michalick MS, Tafuri WL. 2004. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immunol Methods 292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Ouellette M, Fase-Fowler F, Borst P. 1990. The amplified H circle of methotrexate-resistant Leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J 9:1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this paper may be found at https://doi.org/10.6084/m9.figshare.11904954.