Figure 20.
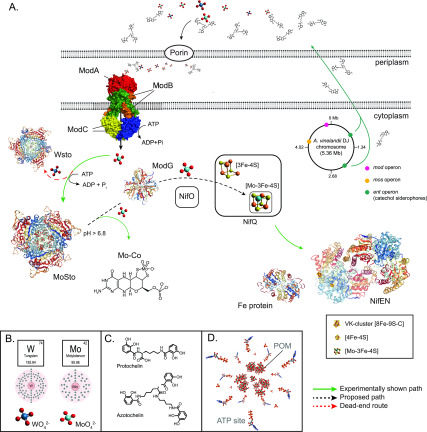
Mo uptake, storage, and processing for nitrogenase in A. vinelandii. (A) Scheme summarizing current understanding of molybdenum trafficking in A. vinelandii. Molybdate in the medium is chelated by siderophores protochelin and azotochelin. Internalization to the periplasm space is believed to occur via outer membrane porins. Active transport of molybdate (and tungstate) to the cytoplasm is mediated by ABC transporters composed of ModABC polypeptides. Most molybdate in A. vinelandii is stored at MoSto complex in a process energized by ATP hydrolysis. However, in Mo-starved A. vinelandii cells, MoSto can store tungstate forming WSto.186 Mo in MoSto is available to the molybdoenzymes nitrogenase and nitrate reductase. Pathway branching appears to occur before involvement of ModG, a trimer that binds eight molybdates at the interface of its subunits. NifO is also involved in directing Mo toward FeMo-co against the Mo-co branch. NifQ carries a [Mo-3Fe-4S] cluster shown to deliver Mo to the NifEN scaffold protein where it will be incorporated into an Fe–S cluster precursor to generate FeMo-co. The structures of a ModA2B2C2 transporter (PDB: 2ONK), the α3β3 MoSto (PDB: 6GU5) and WSto (PDB: 2OGX), an α3 ModG (PDB 1H9M), α2β2 NifEN (PDB: 3PDI), and the Fe protein (PDB: 1NIP) are shown. Structure images created with NGL viewer36 and RCSB PDB. (B) Molybdenum and tungsten electronic shells and their molybdate and tungstate forms. (C) Common A. vinelandii metalophores. (D) Inside details of the MoSto protein crystal structure shown in panel A including POMs (molybdenum-based polyoxometalates) and ATP sites.