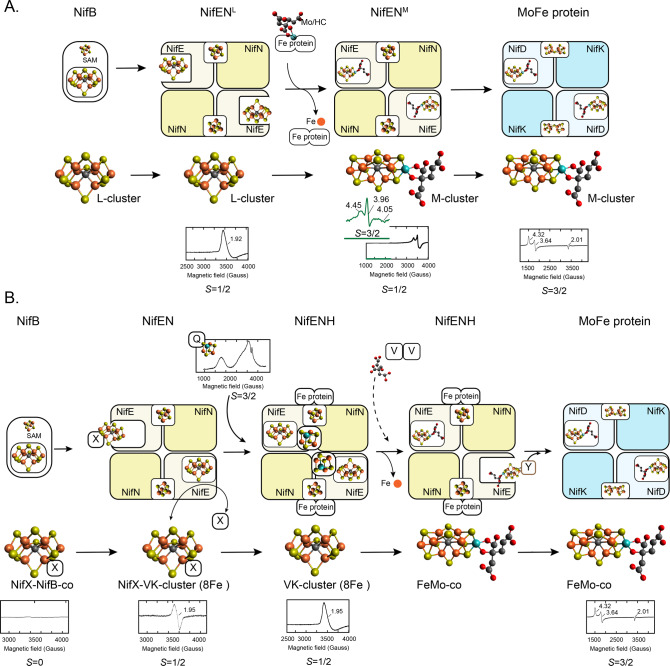
Figure 25.
Two models for NifEN metal cluster composition and Mo incorporation into an Fe–S cluster FeMo-co precursor to mature the cofactor within NifEN. Each model shows the protein and metal cluster nomenclature used. EPR signals used as spectroscopic evidence of protein-bound FeMo-co, FeMo-co precursors, and other MoFeS clusters are shown. (A) Model in which the product of NifB, called L-cluster, is delivered by NifB to apo-NifEN via direct interaction to generate the NifENL form. In subsequent step, the Fe protein delivers a Mo-homocitrate complex that replaces one terminal Fe atom in the L-cluster to generate the M-cluster found in a NifENM species. The M-cluster is transferred from NifENM to apo-MoFe protein by direct interaction to generate active MoFe protein. (B) Model in which the product of NifB, called NifB-co, is transferred to apo-NifEN via NifX. Within NifEN, NifB-co is rapidly transformed into the VK-cluster by NifEN. VK-cluster can be extracted from NifEN by NifX. Both NifX/NifB-co and NifX/VK-cluster complexes have been spectroscopically analyzed. In addition to the VK-cluster, NifEN contains a [Mo-3Fe-4S] cluster proposed to be donated by NifQ, which carries a similar cluster. Homocitrate is donated by NifV. Once all FeMo-co precursors are bound to NifEN, the docked Fe protein exerts a conformational change in NifEN that promotes FeMo-co formation. Mature FeMo-co is transferred to apo-MoFe protein via NafY. NifQ inset adapted from ref (73). Copyright 2008 PNAS.