Abstract
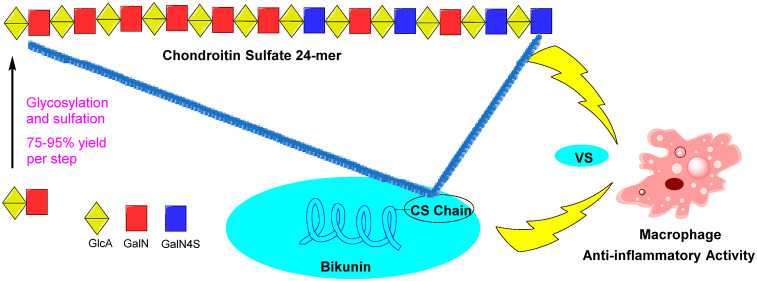
Bikunin, a chondroitin sulfate (CS) proteoglycan clinically used to treat acute inflammation and sepsis, contains a CS chain with more than 20 monosaccharide units. To understand the function of the CS chain of bikunin, synthesis of long CS chains is needed. After exploring multiple glycosylation approaches and protective group chemistry, we report herein the successful generation of the longest CS chain to date (24-mer) in an excellent overall yield on a multi-mg scale. The anti-inflammatory activities of both bikunin and the synthetic 24-mer were determined, and the results demonstrate that both the glycan and the core protein are important for anti-inflammatory activities of bikunin by reducing macrophage production of proinflammatory cytokines.
Short abstract
The synthesis of the longest chondroitin sulfate chain (24-mer) to date is reported. The glycan and the core protein are both important for bioactivities of a chondroitin sulfate proteoglycan, bikunin.
Introduction
Bikunin, also known as the urinary trypsin inhibitor, is a glycoprotein in human plasma and urine.1 Bikunin has many biological functions and is one of the main anti-inflammatory mediators.2−4 The levels of bikunin in plasma and urine can be significantly increased (up to 10-fold) for a range of conditions, including cancer and chronic inflammation.2,3,5,6 Clinically, bikunin has been used as a drug to treat acute inflammatory disorders such as pancreatitis, septic shock, and disseminated intravascular coagulation.
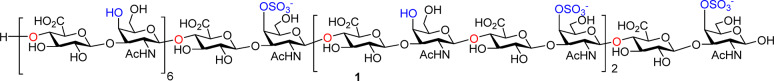
Bikunin is a proteoglycan consisting of a 147 amino acid residue core protein with a chondroitin sulfate (CS) chain attached to serine-10.7,8 Linhardt and co-workers have performed groundbreaking sequencing studies of bikunin CS chains using Fourier transform ion cyclotron resonance mass spectrometry.9 The CS chain of bikunin was determined to contain over 20 monosaccharide units with repeating glucuronic acid (GlcA) and N-acetyl galactosamine (GalNAc).9−11 Only 4-OH groups of GalNAc are partially sulfated in bikunin associated CS and 4–7 O-sulfate groups were found positioned toward the reducing end of the CS chain.3,9−13 Herein, we report the first synthesis of a CS chain 24-mer 1 from bikunin (Figure 1) and its anti-inflammatory activities. The 24-mer is the longest CS chain synthesized to date. Biological studies showed that the CS chain is important for the anti-inflammatory effect of bikunin, while the core protein without the CS chain actually enhanced the levels of inflammatory cytokine secreted from macrophages treated with lipopolysaccharide (LPS).
Figure 1.
Structure of bikunin CS chain (24-mer) 1.
Innovative technologies have been developed toward the synthesis of defined CS oligosaccharides,14,15 which include enzymatic synthesis,16 synthesis with building blocks derived from natural polysaccharides,17−20 automated solid phase synthesis,21,22 and small libraries of CS with varying sulfation patterns.23−28 The longest synthetic defined CS glycans produced to date were octasaccharides23 and nonasaccharides.16,19 To assemble a long CS chain such as 24-mer 1, new synthetic strategies need to be established.
Results and Discussion
Synthesis of Fully Protected CS 24-mer
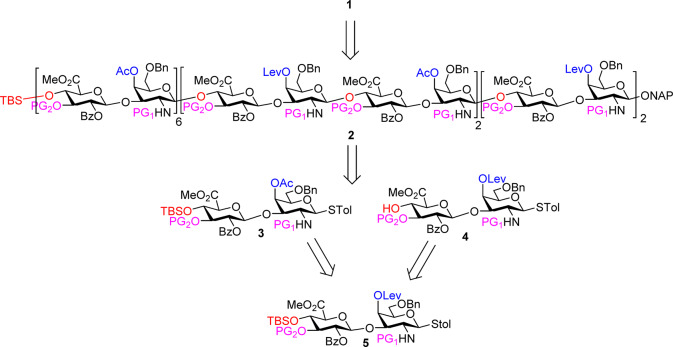
We envision that 24-mer 1 can be obtained from the fully protected precursor 24-mer 2, which in turn can be generated using disaccharide building blocks 3 and 4. The 4-OH groups of the reducing end galactosamine unit of both 3 and 4 are differentially protected for future selective sulfation. The disaccharides 3 and 4 can serve as both a glycosyl donor and an acceptor, which can be derived from the common precursor 5 (Scheme 1). A key in the synthesis is selection of the protective group on the amine, which should facilitate the formation of the desired 1,2-trans glycosides and be deprotected in high yields as there would be 12 such groups to be removed to generate the 24-mer 1. In addition, it was important to use the tbutyldimethylsilyl (TBS) group as the protective group on 4-OH of glucuronic acid (e.g., donor 3), as the presence of TBS helped enhance the solubility of the building blocks for high-yield glycosylation and the ease in purification.
Scheme 1. Retrosynthetic Design of 24-mer 1.
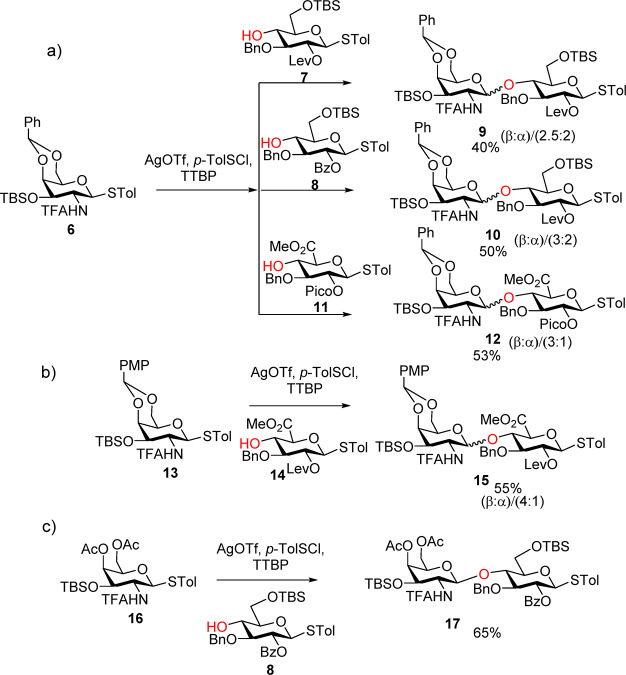
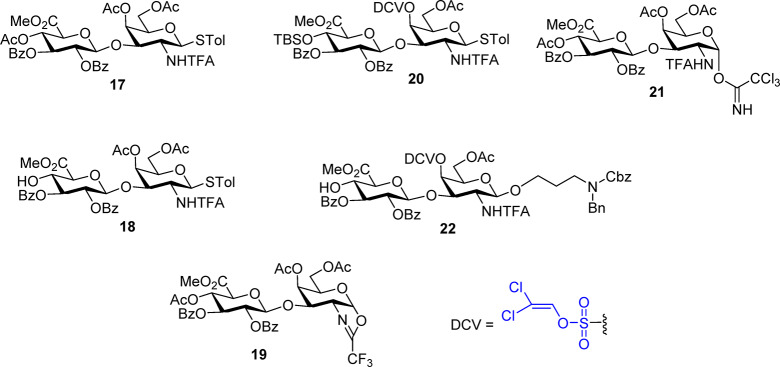
We started our synthesis by testing trifluoroacetamide (TFA)27,29 protected galactosamine (GalN) monosaccharide as the donor due to the potential ease of TFA removal under a mild basic condition. However, the glycosylation between GalNTFA donor 6 and glucoside acceptor 7 or 8 gave disaccharides 9 and 10 as anomeric mixtures with little β-selectivity (β:α = 2.5:2, 3:2, respectively) (Scheme 2a). Switching the acceptor to the glucuronic acid derivative 11 or the donor to the more electron donating p-methoxybenzylidene protected GalNTFA 13 led to modest improvements (Schemes 2a,b). Interestingly, replacing the 4,6-benzylidene in the donor with di-4,6-O-acetates (donor 16) produced the desired β-linked disaccharide 17 as the only anomer isolated (Scheme 2c). These suggest that the 4,6-benzylidene moiety likely restricted the conformational freedom of the pyranoside ring upon donor activation, rendering it difficult for TFA to assist the β-glycoside formation by neighboring group participation.
Scheme 2. Challenges Encountered Using TFA Protected GalN Monosaccharide as Donors.
(a) Stereochemical challenges in formation of disaccharides 9, 10, and 12 from donor 6. (b) Modest stereoselectivity observed in the formation of disaccharide 15 from donor 13. (c) Formation of 17 in high stereoselectivity from donor 16.
With the success of using 16 as the glycosyl donor, we examined the utility of disaccharide (Figure 2) donors such as 17 containing NHTFA protected galactosamine at the reducing end. Unfortunately, glycosylation of 17 with disaccharide acceptor 18 did not yield the desired tetrasaccharide, with the oxazoline 19 formed as the major side product in 55% yield. Changes of protective groups (donor 20), anomeric leaving group (21) on the donor, as well as the acceptor structure (22) did not lead to productive glycosylation either, with the donor mostly converted to the corresponding disaccharide oxazoline side product. The failures of disaccharide donors such as 20 and 17 are possibly because of their lower anomeric reactivities compared to monosaccharide 16 due to the electron withdrawing effect of the additional glycan ring30 leading to enhanced stabilities of the oxazoline intermediates.
Figure 2.
Structures of disaccharide building blocks examined containing NHTFA protected galactosamine.
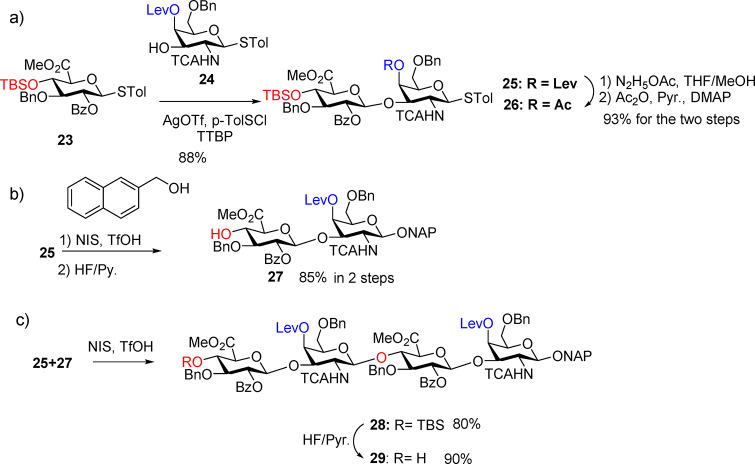
To overcome the challenges encountered with TFA protected disaccharide donors, we envision that the reactivities of building blocks can be enhanced by using less electron withdrawing protective groups such as the trichloroacetyl (TCA)14,17,18,26,31−33 and benzyl groups. Thus, glucoside donor 23 and TCA protected GalN acceptor 24 were designed and synthesized. Preactivation of donor 23 by p-TolSCl/AgOTf34 followed by the addition of acceptor 24 produced the key disaccharide building block GlcA-GalN 25 in an excellent 88% yield (Scheme 3a). To analyze the ability of TCANH bearing disaccharide 25 as a glycosyl donor, glycosylation of 25 with 2-naphthol was carried out first, which, with subsequent TBS cleavage, afforded disaccharide 27 in 85% yield for the two steps (Scheme 3b). Furthermore, coupling between 25 and acceptor 27 generated the desired tetrasaccharide 28 in 80% yield (Scheme 3c), suggesting that disaccharide 25 is a suitable glycosyl donor for CS synthesis. Deprotection of the TBS group on 27 produced the tetrasaccharide 29 in an excellent 90% yield.
Scheme 3. (a) Synthesis of Disaccharide Donors 25 and 26, (b) Synthesis of Acceptor 27, and (c) Synthesis of Tetrasaccharide Acceptor 29.
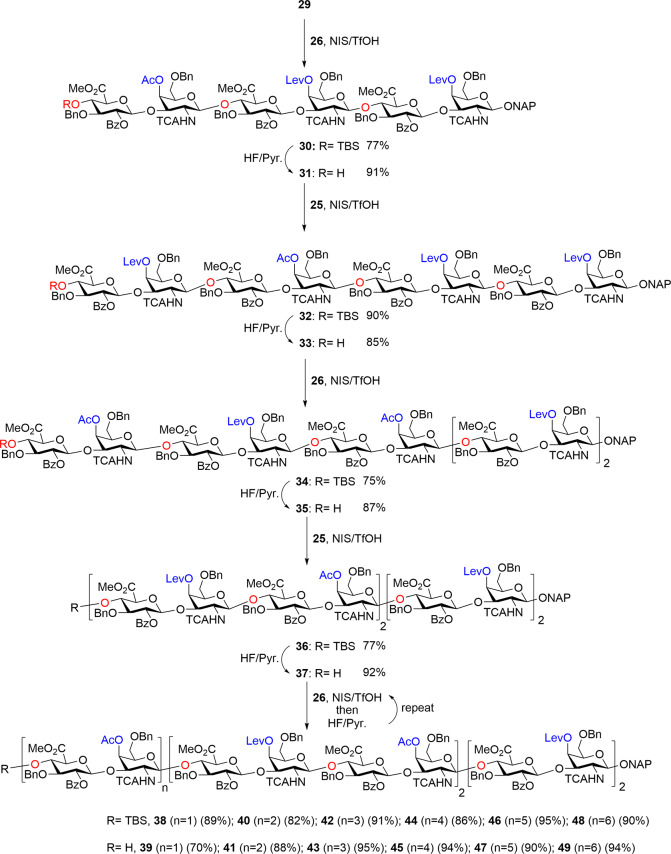
To elongate the CS backbone toward the CS 24-mer, the 4-O acetate bearing disaccharide 26 (Scheme 3a) was obtained as a second disaccharide building block. Glycosylation between donor 26 and tetrasaccharide acceptor 29 proceeded smoothly to give hexasaccharide 30 in 77% yield (Scheme 4). TBS deprotection of 30 followed by glycosylation with levulinyl ester bearing donor 25 gave octasaccharide 32 in an excellent yield (90%) (Scheme 4). The TBS deprotection and glycosylation with donor 26 and 25 were repeated iteratively, producing decasaccharide 34 in 75% yield, dodecasaccharide 36 in 77% yield, tetradecasaccharide 38 in 89% yield, hexadecasaccharide 40 in 82% yield, octadecasaccharide 42 in 91% yield, icosasaccharide 44 in 86% yield, docosasaccharide 46 in 95% yield, and tetracosasaccharide 48 in 90% yield (Scheme 4). This glycosylation approach was readily scalable, generating the protected 24-mer 48 on a 200 mg scale. The TBS group in tetracosasaccharide 48 was deprotected leading to tetracosasaccharide 49, which, with its free OH at the nonreducing end, could serve as an acceptor for further chain elongation if necessary.
Scheme 4. Synthesis of Protected 24-mer 49.
Deprotection of the 24-mer 49
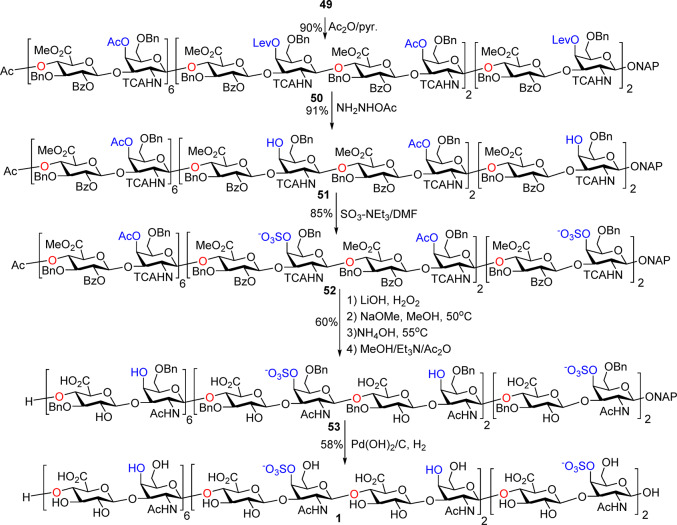
With the fully protected 24-mer 49 in hand, we went forward to deprotection and sulfation (Scheme 5). 49 was acetylated with Ac2O to give tetracosasaccharide 50. Selective cleavage of the Lev groups was performed by treatment of 50 with hydrazine acetate to afford the tetraol 51 in 91% yield, which was subjected for sulfation using sulfur trioxide–triethylamine complex (SO3–NEt3) in dry DMF to provide the sulfated 24-mer 52 in 85% yield.
Scheme 5. Deprotection and Sulfation of Protected 24-mer 55 Successfully Generated CS 24-mer 1.
The next challenging task is the unprecedented conversion of 12 NHTCAs to acetamides. The Zn–Cu couple reduction method33,35 was tested first on 50, which led to incomplete conversions and glycan decompositions. Next, a basic condition was applied to remove the TCA. 52 was first treated with LiOH (1 M) and H2O231 for deprotection of methyl esters and a multitude of acyl groups. However, this reaction was slow with only partial removal of TCA. Similar phenomena were reported as in the synthesis of hyaluronan decasaccharide; deprotection of the five TCA moieties took 3 weeks.36 Addition of NaOMe to the reaction mixture of 52 with heating at 50 °C did not give the desired fully deprotected product. Increasing the basicity of the reaction led to cleavage of the glycan chain. After extensive screening, we discovered that treatment of the reaction mixture after NaOMe with concentrated ammonia (2 days at 55 °C) could cleanly remove all 12 TCA protective groups as monitored by mass spectrometry (see the SI, page S186). The resulting free amines were selectively N-acetylated to afford 53 in 60% yield over 4 steps from 50. Finally, hydrogenolysis of 53 using Pd(OH)2/C gave the desired 24-mer 1 in 58% yield (Scheme 5).
CS Chain Is Important for the Anti-Inflammatory Activities of Bikunin
With the homogeneous 24-mer CS 1 in hand, we investigated its anti-inflammatory effects using a macrophage/lipopolysaccharide (LPS) assay.37,38 Found on the outer membrane of Gram negative bacteria, LPS can activate macrophages to release a large amount of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), which may lead to septic shock, a potentially fatal medical condition resulting from infections.39 To better understand the structural requirement of bikunin on its inflammatory responses, bikunin was treated with chondroitinase ABC to cleave off the endogenous CS chain producing the core protein lacking the CS chain.40 In addition, bikunin was treated with actinase E to completely digest the core protein backbone generating the bikunin associated CS chain, which mainly consists of CS-A9 as confirmed by our NMR and MS analysis. The abilities of macrophages to respond to LPS upon incubation with bikunin, core protein, synthetic CS 24-mer 1, commercially available CS-A polysaccharide, and bikunin CS were determined by quantifying the amounts of TNF-α released from macrophages using an enzyme linked immunosorbent assay (ELISA) (Figure 3). Consistent with its clinical application, bikunin was able to reduce the amounts of TNF-α from macrophages induced by LPS in a dose dependent manner (Figure 3).38,41 At 5 and 50 μM of bikunin, the levels of TNF-α were reduced to 65% and 39% of those from macrophages not receiving bikunin. Interestingly, under the same condition, macrophages treated with the core protein without CS produced more TNF-α, suggesting that the core protein alone actually exacerbated macrophage inflammations. In contrast to the core protein, commercial CS-A polysaccharide and bikunin CS were able to reduce TNF-α levels to 80% at 5 μM concentration. The synthetic CS 24-mer 1 exhibited improved anti-inflammatory effects compared to both CS-A polysaccharide and bikunin CS. At a low dose (5 μM) of CS 24-mer 1, the TNF-α level from treated macrophages decreased to 60% of the control, similar to that by bikunin. The further increase of the doses of CS-24-mer 1 did not lead to significant differences in reduction of TNF-α levels (Figure 3). Our results suggest that the CS chain could modify the function of bikunin core protein, leading to anti-inflammatory activities against macrophages.
Figure 3.
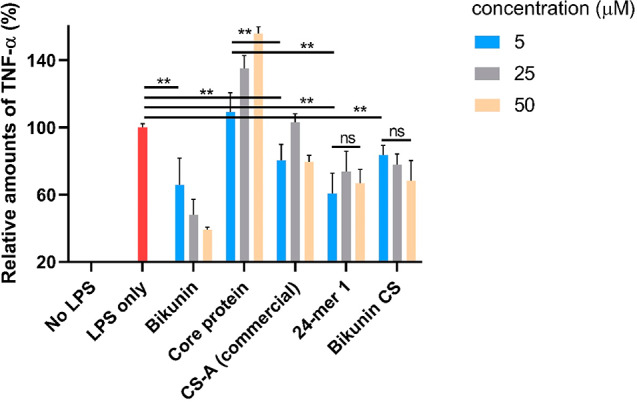
Amounts of TNF-α secreted by macrophage raw 264.7 cells upon treatment with LPS (100 ng/mL) in the presence of bikunin, bikunin core protein, CS-A polysaccharide, bikunin CS, and the synthetic CS 24-mer 1 at 5, 25, and 50 μM concentrations, respectively. The amounts of TNF-α secreted by macrophages were quantified by ELISA and normalized against the levels of TNF-α from cells treated with LPS only. Each experiment was performed at least three times with the mean values presented. The error bars represent standard deviations of the measurements. Statistical analyses were performed through a two-tailed t test using GraphPad Prism software. ** p < 0.01. ns: no significant differences.
A possible reason for the absence of dose dependent responses in the anti-inflammatory activities from CS polysaccharide or the synthetic CS 24-mer is that CS and bikunin may have different aggregation states. To test this, we measured hydrodynamic diameters of CS and bikunin in solution by dynamic light scattering (DLS), and both CS and bikunin showed small size decreases at higher concentrations, possibly due to increased repulsive interactions between polymer chains (Figure S2). Furthermore, little UV–vis absorbance at 600 nm was observed with aqueous solutions of CS or bikunin at all concentrations. These results suggest that CS and bikunin are soluble and not aggregating at concentrations studied. As solubility and aggregation are not likely the main factor, the differential dose dependent responses may be due to different cellular receptors of CS from that of bikunin for cellular response or transport, which will require further investigation.
Conclusion
In conclusion, due to the structural heterogeneity of CS polysaccharide from nature,2,9,42 it is important to develop synthetic methodologies to access well-defined CS chains. While multiple innovative syntheses of CS oligosaccharides up to nonasaccharides have been reported, the need to study CS proteoglycans such as bikunin requires longer CS chains. Herein, we report that by judicious design of building blocks and protective groups, fully protected CS 24-mer could be prepared via chemical glycosylation in a high overall yield on a 200 mg scale. Deprotection and sulfation conditions have been established to remove the multitude of protective groups and install the requisite O-sulfates producing the CS 24-mer 1, which is the longest CS chain synthesized to date.
As bikunin is an approved drug to treat inflammatory conditions, the abilities of the CS 24-mer 1 to reduce the inflammatory effects of macrophages were studied and compared with bikunin glycoprotein and the corresponding core protein backbone. Interestingly, the core protein of bikunin lacking the endogenous CS chain was found to stimulate the production of TNF-α, a powerful proinflammatory cytokine, from macrophages treated with LPS. In contrast, CS 24-mer 1 exhibited anti-inflammatory activities by reducing the levels of TNF-α from macrophages, and the level of reduction was similar to that obtained using the full bikunin glycoprotein at 5 μM concentrations of bikunin. The similar activities observed of synthetic CS 24-mer compared to bikunin CS suggest the synthetic 24-mer can recapitulate the function of native CS polysaccharides. The ability of obtaining long CS chains opens up avenues to investigate biological functions of CS with its native length in glycoproteins. Studies are underway to synthesize such glycopeptides and glycoproteins and investigate their multifaceted biological functions.
Acknowledgments
We are grateful for the financial support from the National Institute of General Medical Sciences, NIH (R01GM072667).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.9b01199.
Detailed experimental procedures, characterization data of all the synthesized compounds and intermediates, NMR spectra (1H, 13C, COSY, HSQC, HMBC), and MS (ESI-MS or MALDI-MS) spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Proksch G. J.; Routh J. I. The purification of the trypsin inhibitor from human pregnancy urine. Transl. Res. 1972, 79, 491–499. [PubMed] [Google Scholar]
- Pugia M. J.; Valdes R.; Jortani S. A. Bikunin (urinary trypsin inhibitor): structure, biological relevance, and measurement. Adv. Clin. Chem. 2007, 44, 223–245. 10.1016/S0065-2423(07)44007-0. [DOI] [PubMed] [Google Scholar]
- Fries E.; Blom A. M. Bikunin — not just a plasma proteinase inhibitor. Int. J. Biochem. Cell Biol. 2000, 32, 125–137. 10.1016/S1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- Maehara K.; Kanayama N.; Halim A.; Elmaradny E.; Oda T.; Fujita M.; Terao T. Down-regulation of interleukin-8 gene expression in HL60 cell line by human kunitz-type trypsin inhibitor. Biochem. Biophys. Res. Commun. 1995, 206, 927–934. 10.1006/bbrc.1995.1131. [DOI] [PubMed] [Google Scholar]
- Lin S. D.; Endo R.; Kuroda H.; Kondo K.; Miura Y.; Takikawa Y.; Kato A.; Suzuki K. Plasma and urine levels of urinary trypsin inhibitor in patients with chronic liver diseases and hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2004, 19, 327–332. 10.1111/j.1440-1746.2003.03221.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H.; Kobayashi H.; Yagyu T.; Wakahara K.; Kondo T.; Kurita N.; Sekino H.; Inagaki K.; Suzuki M.; Kanayama N.; Terao T. Plasma bikunin as a favorable prognostic factor in ovarian cancer. J. Clin. Oncol. 2005, 23, 1463–1472. 10.1200/JCO.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Zhuo L.; Hascall V. C.; Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J. Biol. Chem. 2004, 279, 38079–38082. 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Carr P. D.; Guss J. M.; Ollis D. L. The crystal structure of bikunin from the inter-α-inhibitor complex: a serine protease inhibitor with two Kunitz domains. J. Mol. Biol. 1998, 276, 955–966. 10.1006/jmbi.1997.1582. [DOI] [PubMed] [Google Scholar]
- Ly M.; Leach F. E. III; Laremore T. N.; Toida T.; Amster I. J.; Linhardt R. J. The proteoglycan bikunin has a defined sequence. Nat. Chem. Biol. 2011, 7, 827–833. 10.1038/nchembio.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enghild J. J.; Thøgersen I. B.; Cheng F.; Fransson L.-Å.; Roepstorff P.; Rahbek-Nielsen H. Organization of the inter-α-inhibitor heavy chains on the chondroitin sulfate originating from ser10 of bikunin: posttranslational modification of IαI-derived bikunin. Biochemistry 1999, 38, 11804–11813. 10.1021/bi9908540. [DOI] [PubMed] [Google Scholar]
- Capon C.; Mizon C.; Lemoine J.; Rodié-Talbère P.; Mizon J. In acute inflammation, the chondroitin-4 sulphate carried by bikunin is not only longer; it is also undersulphated. Biochimie 2003, 85, 101–107. 10.1016/S0300-9084(03)00066-X. [DOI] [PubMed] [Google Scholar]
- Toyoda H.; Kobayashi S.; Sakamoto S.; Toida T.; Imanari T. Structural analysis of a low-sulfated chondroitin sulfate chain in human urinary trypsin inhibitor. Biol. Pharm. Bull. 1993, 16, 945–947. 10.1248/bpb.16.945. [DOI] [PubMed] [Google Scholar]
- Yamada S.; Oyama M.; Kinugasa H.; Nakagawa T.; Kawasaki T.; Nagasawa S.; Khoo K.-H.; Morris H. R.; Dell A.; Sugahara K. The sulphated carbohydrate-protein linkage region isolated from chondroitin 4-sulphate chains of inter-α-trypsin inhibitor in human plasma. Glycobiology 1995, 5, 335–341. 10.1093/glycob/5.3.335. [DOI] [PubMed] [Google Scholar]
- Vibert A.; Jacquinet J. C.; Lopin-Bon C. Recent advances in the chemical and enzymatic chondroitin sulfate synthesis. J. Carbohydr. Chem. 2011, 30, 393–414. 10.1080/07328303.2011.619286. [DOI] [Google Scholar]
- Ramadan S.; Yang W.; Huang X. Chapter 8. Synthesis of chondroitin sulfate oligosaccharides and chondroitin sulfate glycopeptides. Synthetic Glycomes: The Royal Society of Chemistry 2019, 172–206. and references cited therein 10.1039/9781788016575-00172. [DOI] [Google Scholar]
- Li J.; Su G.; Liu J. Enzymatic synthesis of homogeneous chondroitin sulfate oligosaccharides. Angew. Chem., Int. Ed. 2017, 56, 11784–11787. 10.1002/anie.201705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert A.; Lopin-Bon C.; Jacquinet J.-C. From polymer to size-defined oligomers: a step economy process for the efficient and stereocontrolled construction of chondroitin oligosaccharides and biotinylated conjugates thereof: part 1. Chem. - Eur. J. 2009, 15, 9561–9578. 10.1002/chem.200900740. [DOI] [PubMed] [Google Scholar]
- Lopin C.; Jacquinet J.-C. From polymer to size-defined oligomers: an expeditious route for the preparation of chondroitin oligosaccharides. Angew. Chem., Int. Ed. 2006, 45, 2574–2578. 10.1002/anie.200503551. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Liu H.; Lin L.; Yao W.; Zhao J.; Wu M.; Li Z. Synthesis of fucosylated chondroitin sulfate nonasaccharide as a novel anticoagulant targeting intrinsic factor xase complex. Angew. Chem., Int. Ed. 2018, 57, 12880–12885. 10.1002/anie.201807546. [DOI] [PubMed] [Google Scholar]
- Chng Y. S.; Tristan G.; Yip G. W.; Lam Y. Protecting-group-free synthesis of chondroitin 6-sulfate disaccharide and tetrasaccharide. Org. Lett. 2019, 21, 4559–4562. 10.1021/acs.orglett.9b01457. [DOI] [PubMed] [Google Scholar]
- Eller S.; Collot M.; Yin J.; Hahm H. S.; Seeberger P. H. Automated solid-phase synthesis of chondroitin sulfate glycosaminoglycans. Angew. Chem., Int. Ed. 2013, 52, 5858–5861. 10.1002/anie.201210132. [DOI] [PubMed] [Google Scholar]
- Liang C. F.; Hahm H. S.; Seeberger P. H. Automated synthesis of chondroitin sulfate oligosaccharides. Methods Mol. Biol. 2015, 1229, 3–10. 10.1007/978-1-4939-1714-3_1. [DOI] [PubMed] [Google Scholar]
- Tamura J.; Nakada Y.; Taniguchi K.; Yamane M. Synthesis of chondroitin sulfate E octasaccharide in a repeating region involving an acetamide auxiliary. Carbohydr. Res. 2008, 343, 39–47. 10.1016/j.carres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Matsushita K.; Nakata T.; Takeda-Okuda N.; Nakanaka S.; Kitagawa H.; Tamura J. Synthesis of chondroitin sulfate CC and DD tetrasaccharides and interactions with 2H6 and LY111. Bioorg. Med. Chem. 2018, 26, 1016–1025. 10.1016/j.bmc.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Miyachi K.; Wakao M.; Suda Y. Syntheses of chondroitin sulfate tetrasaccharide structures containing 4,6-disulfate patterns and analysis of their interaction with glycosaminoglycan-binding protein. Bioorg. Med. Chem. Lett. 2015, 25, 1552–1555. 10.1016/j.bmcl.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Gama C. I.; Tully S. E.; Sotogaku N.; Clark P. M.; Rawat M.; Vaidehi N.; Goddard W. A. 3rd; Nishi A.; Hsieh-Wilson L. C. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2006, 2, 467–473. 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- Macchione G.; Maza S.; Kayser M. M.; de Paz J. L.; Nieto P. M. Synthesis of chondroitin sulfate oligosaccharides using N-(tetrachlorophthaloyl)- and N-(trifluoroacetyl)galactosamine building blocks. Eur. J. Org. Chem. 2014, 2014, 3868–3884. 10.1002/ejoc.201402222. [DOI] [Google Scholar]
- Yang S.; Liu Q.; Zhang G.; Zhang X.; Zhao Z.; Lei P. An approach to synthesize chondroitin sulfate-E (CS-E) oligosaccharide precursors. J. Org. Chem. 2018, 83, 5897–5908. 10.1021/acs.joc.8b00157. [DOI] [PubMed] [Google Scholar]
- Maza S.; Mar Kayser M.; Macchione G.; López-Prados J.; Angulo J.; de Paz J. L.; Nieto P. M. Synthesis of chondroitin/dermatan sulfate-like oligosaccharides and evaluation of their protein affinity by fluorescence polarization. Org. Biomol. Chem. 2013, 11, 3510–3525. 10.1039/c3ob40306h. [DOI] [PubMed] [Google Scholar]
- Koeller K. M.; Wong C.-H. Synthesis of complex carbohydrates and glycoconjugates: enzyme-based and programmable one-pot strategies. Chem. Rev. 2000, 100, 4465–4493. 10.1021/cr990297n. [DOI] [PubMed] [Google Scholar]
- Tully S. E.; Mabon R.; Gama C. I.; Tsai S. M.; Liu X.; Hsieh-Wilson L. C. A chondroitin sulfate small molecule that stimulates neuronal growth. J. Am. Chem. Soc. 2004, 126, 7736–7737. 10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- Coutant C.; Jacquinet J. C. 2-Deoxy-2-trichloroacetamido-D-glucopyranose derivatives in oligosaccharide synthesis - from hyaluronic-acid to chondroitin 4-sulfate trisaccharides. J. Chem. Soc., Perkin Trans. 1 1995, 1573–1581. 10.1039/p19950001573. [DOI] [Google Scholar]
- Ramadan S.; Yang W.; Zhang Z.; Huang X. Synthesis of chondroitin sulfate A bearing syndecan-1 glycopeptide. Org. Lett. 2017, 19, 4838–4841. 10.1021/acs.orglett.7b02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Huang L.; Wang H.; Ye X.-S. Iterative one-pot synthesis of oligosaccharides. Angew. Chem., Int. Ed. 2004, 43, 5221–5224. 10.1002/anie.200460176. [DOI] [PubMed] [Google Scholar]
- Vibert A.; Lopin-Bon C.; Jacquinet J.-C. Efficient alternative for the reduction of N-trichloroacetyl groups in synthetic chondroitin oligosaccharide intermediates. Tetrahedron Lett. 2010, 51, 1867–1869. 10.1016/j.tetlet.2010.02.005. [DOI] [Google Scholar]
- Lu X.; Kamat M.; Huang L.; Huang X. Chemical synthesis of a hyaluronic acid decasaccharide. J. Org. Chem. 2009, 74, 7608–7617. 10.1021/jo9016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakahara K.; Kobayashi H.; Yagyu T.; Matsuzaki H.; Kondo T.; Kurita N.; Sekino H.; Inagaki K.; Suzuki M.; Kanayama N.; Terao T. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J. Infect. Dis. 2005, 191, 930–938. 10.1086/428134. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H.; Kobayashi H.; Yagyu T.; Wakahara K.; Kondo T.; Kurita N.; Sekino H.; Inagaki K.; Suzuki M.; Kanayama N.; Terao T. Bikunin inhibits lipopolysaccharide-induced tumor necrosis factor alpha induction in macrophages. Clin. Diagn. Lab. Immunol. 2004, 11, 1140–1147. 10.1128/CDLI.11.6.1140-1147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D.; Bellissant E.; Cavaillon J.-M. Septic shock. Lancet 2005, 365, 63–78. 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- Lord M. S.; Day A. J.; Youssef P.; Zhuo L.; Watanabe H.; Caterson B.; Whitelock J. M. Sulfation of the bikunin chondroitin sulfate chain determines heavy chain.hyaluronan complex formation. J. Biol. Chem. 2013, 288, 22930–22941. 10.1074/jbc.M112.404186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama S.; Yamada Y.; Onogi A.; Shigetomi H.; Ueda S.; Tsuji Y.; Haruta S.; Kawaguchi R.; Yoshida S.; Sakata M.; Sado T.; Kitanaka T.; Oi H.; Yagyu T.; Kobayashi H. Bikunin suppresses expression of pro-inflammatory cytokines induced by lipopolysaccharide in neutrophils. J. Endotoxin Res. 2007, 13, 369–376. 10.1177/0968051907086464. [DOI] [PubMed] [Google Scholar]
- Nadanaka S.; Clement A.; Masayama K.; Faissner A.; Sugahara K. Characteristic hexasaccharide sequences in octasaccharides derived from shark cartilage chondroitin sulfate D with a neurite outgrowth promoting activity. J. Biol. Chem. 1998, 273, 3296–3307. 10.1074/jbc.273.6.3296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.