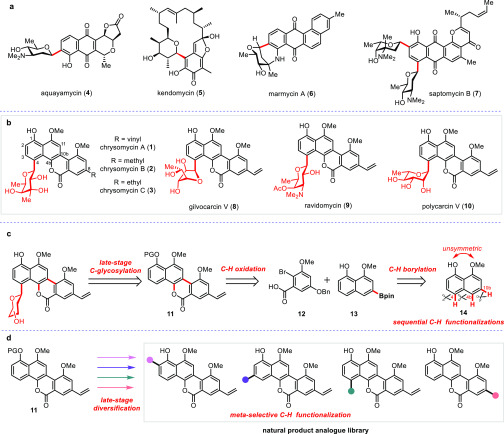
Figure 1.
C-aryl glycoside natural products and synthetic plans for chrysomycin A and its analogues. (a) Representative bioactive C-aryl glycosides. (b) Structures of gilvocarcin family natural products. (c) Our bond disconnections of chrysomycin A. In our retrosynthetic analysis of chrysomycin A, both the sugar moiety and vinyl group were assembled at the late stage. The core structure of the chromophore was constructed through sequential regioselective C–H functionalizations. (d) Late-stage diversification of the natural product at multiple sites.