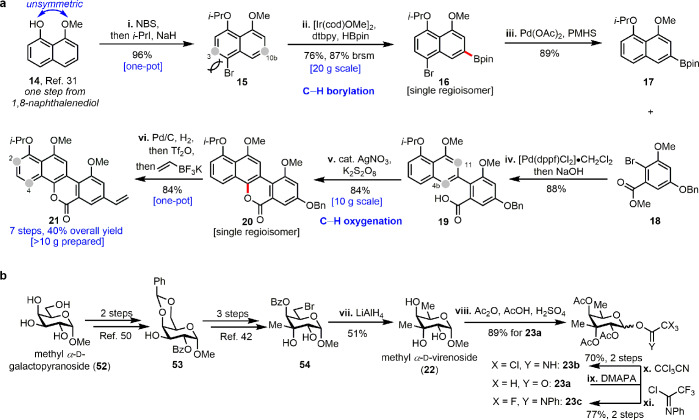
Figure 2.
Concise and scalable syntheses of aglycon and glycosyl donors. (a) Regioselective C–H functionalizations enabled scalable preparation of the aglycon 21. (b) Synthesis of glycosyl donors 23a–c. Reagents and conditions are as follows: (i) NBS, MeCN, r.t.; i-PrI, NaH, DMF, 0 to 70 °C. (ii) [Ir(cod)OMe]2, dtbpy, HBpin, hexane, 80 °C. (iii) Pd(OAc)2, KF, PMHS, THF, H2O, r.t. (iv) [Pd(dppf)Cl2]·CH2Cl2, KOH, MTBE/H2O, 80 °C, then 40% NaOH (aq). (v) K2S2O8, AgNO3, MeCN/H2O, 50 °C. (vi) Pd/C, H2, MeOH, r.t.; Tf2O, Et3N, CH2Cl2, −78 °C; potassium vinyltrifluoroborate, [Pd(dppf)Cl2]·CH2Cl2, Et3N, n-PrOH, reflux. (vii) LiAlH4, THF, 50 °C. (viii) Ac2O, AcOH, H2SO4, r.t. (ix) DMAPA, THF, 20 °C. (x) CCl3CN, DBU, 4 Å MS, CH2Cl2, r.t. (xi) N-Phenyltrifluoroacetimidoyl chloride, K2CO3, acetone, r.t.