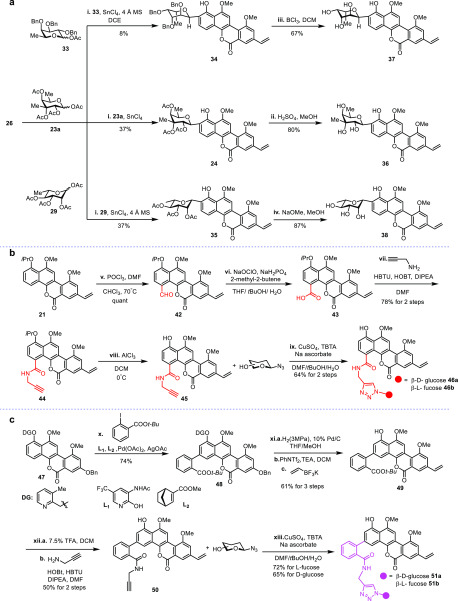
Figure 5.
Late-stage diversification of chrysomycin A. (a) Synthesis of the C2 glycosylated derivatives. (b) Synthesis of the C4 hybrid derivatives. (c) Synthesis of the C3 hybrid derivatives via meta-selective C–H functionalization. Reagents and conditions are as follows: (i) SnCl4, 4 Å MS, DCE, r.t. (ii) 1.5 M H2SO4 in MeOH, 70 °C. (iii) BCl3, CH2Cl2, −78 °C. (iv) NaOMe, MeOH, r.t. (v) POCl3, DMF, CHCl3, 70 °C. (vi) NaOClO, NaH2PO4, 2-methyl-2-butene, THF/tBuOH/H2O, r.t. (vii) 2-Propynylamine, HBTU, HOBt, DIPEA, DMF, r.t. (viii) AlCl3, DCM, r.t. (ix) CuSO4, TBTA, sodium ascorbate, DMF/tBuOH/H2O, r.t. (x) tert-butyl 2-iodobenzoate, L1, L2, Pd(OAc)2, AgOAc, r.t. (xi) Pd/C, H2(3 MPa), THF/MeOH, r.t.; PhNTf2, TEA, DCM, .r.t.; potassium vinyltrifluoroborate, [Pd(dppf)Cl2]·CH2Cl2, Et3N, n-PrOH, reflux. (xii) 7.5% TFA, DCM, r.t.; prop-2-yn-1-amine, HOBT, HBTU, DIPEA, DMF, r.t. (xiii) β-Glycosyl azides, CuSO4, TBTA, Na ascorbate, DMF/tBuOH/H2O, r.t.