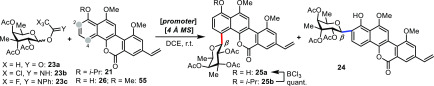
Table 1. Optimization of the C-Glycosylation Reaction.
| entrya | D/A | promoter (equiv) | T (°C) | 4 Å MS (wt equiv) | 25/24b | yield of 25c (%) |
|---|---|---|---|---|---|---|
| 1d | 23a/21 | SnCl4 (9.0) | 25 | 0 | 0/100 | 41e |
| 2 | 23a/21 | SnCl4 (3.0) | 25 | 0 | <5 | |
| 3 | 23a/21 | Et2O·BF3 (3.0) | 25 | 4.0 | 0 | |
| 4 | 23a/21 | TMSOTf (3.0) | 25 | 4.0 | <5 | |
| 5 | 23a/21 | Cp2ZrCl2 (3.0), AgClO4 (3.0) | 25 | 4.0 | 0 | |
| 6 | 23a/21 | SnCl4 (3.0), AgClO4 (3.0) | 25 | 4.0 | 60/40 | 14 |
| 7 | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 72/28 | 25 |
| 8 | 23a/21 | SnCl4 (3.0) | 15 | 4.0 | 52/48 | <15 |
| 9 | 23a/21 | SnCl4 (3.0) | 35 | 4.0 | 55/45 | 10 |
| 10f | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 0 | |
| 11g | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 63/37 | 11 |
| 12 | 23a/21 | SnCl4 (3.0) | 25 | 12.0 | 81/19 | 30 |
| 13 | 23a/21 | SnCl4(3.0) | 25 | 20.0 | >95/5 | 50 |
| 14h | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 50/50 | 11 |
| 15 | 23b/21 | SnCl4 (3.0) | 25 | 20.0 | 90/10 | 26 |
| 16 | 23b/21 | TMSOTf (3.0) | 25 | 20.0 | <5 | |
| 17 | 23b/21 | TMSOTf (0.4) | 25 | 20.0 | <5 | |
| 18 | 23c/21 | SnCl4 (3.0) | 25 | 20.0 | >95/5 | 41 |
| 19 | 23c/21 | SnCl4 (0.5) | 25 | 20.0 | >95/5 | 11i |
| 20 | 23a/55 | SnCl4 (3.0) | 25 | 12.0 | <5j | |
| 21d | 23a/26 | SnCl4 (9.0) | 25 | 0 | 0/100 | 37e |
| 22 | 23a/26 | SnCl4 (3.0) | 25 | 4.0 | 0/100 | <5e |
Conditions: 23 (1.0 equiv), aglycon (3.0 equiv), 4 Å MS, promoter (3.0 equiv), solvent (0.017 M), r.t.
Ratio determined by 1H NMR of the crude reaction mixture.
Combined yield of 25a and 25b.
1.0 equiv of aglycon, 4.0 equiv of 23a, and 9.0 equiv of SnCl4 were used.
Isolated yield of 24.
DCE/THF or MeCN was used as solvent.
Concentration = 0.034 M.
1.0 equiv of aglycon, 3.0 equiv of 23a, and 3.0 equiv of SnCl4 were used.
α-25b was also obtained in 19% yield.
Deprotection of 1-hydroxyl group did not happen. D/A = donor/acceptor.