Figure 1.
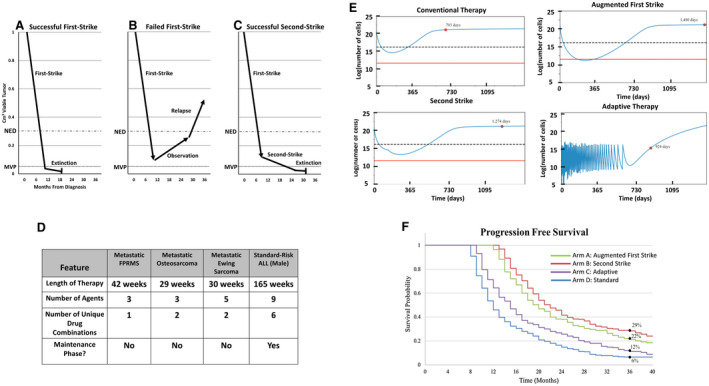
(A) First‐strike therapy reduces disease burden below the threshold of radiographic detection (no evidence of disease [NED]). (B) Minority resistant cell populations are below the NED level, but not the minimum viable population (MVP), driving eventual relapse. (C) With successful second‐strike therapy, the disease burden falls below the MVP, eventually causing extinction (cure). (D) Current sarcoma therapy based on North American protocols contains relatively few agents that are administered for 7 to 9 months. Acute lymphoblastic leukemia (ALL) therapy contains more agents, increased variation in multiagent combinations and dosing, and a prolonged maintenance phase. (E) Simulated in silico outcomes of model parameterized by a conventional chemotherapy setting of an event‐free survival (EFS) of 6% at 3 years are illustrated. Red dots represent EFS for a simulated patient. An augmented first‐strike strategy can affect extinction by reducing levels below a theoretical MVP (indicated by a red line). Second‐strike therapy delays progression but does not increase extinction, whereas adaptive therapy controls progression longer than conventional chemotherapy. A dashed line represents the threshold of detection by imaging. (F) A simulated cohort of patients is illustrated with random variability around key parameters presented as a Kaplan‐Meier curve. Importantly, this is based on a calibration of parameters that generates a 3‐year EFS of 6%. The data that accrue through a proposed clinical trial will update and improve model parameterization and model predictions. These baseline data show an example of how an evolutionary framework can improve therapeutic regimens.
