Figure 2.
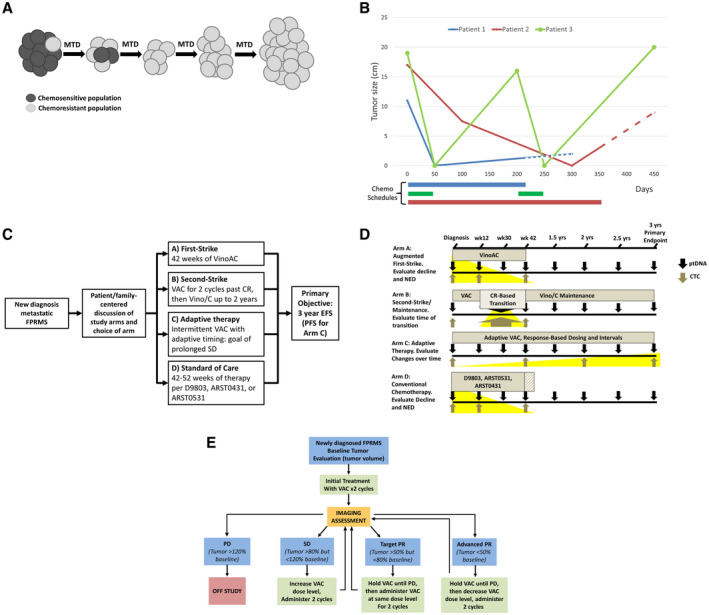
(A) Over time and under selection of continued first‐strike therapy at the maximum tolerated dose (MTD), minor resistant populations emerge, leading to relapse, termed competitive release (adapted from West et al, 201942). (B) An illustration of clinical courses compares MTD therapy with “adaptive” therapy in metastatic, fusion‐positive rhabdomyosarcoma (FPRMS). Data are from aggregate tumor measurements of 3 patients who demonstrated an initial complete response to therapy followed by subsequent progression, with the truncation of lines representing death from disease. Although patients 1 and 2 progressed on therapy and were refractory to second‐line therapies, patient 3 chose to stop therapy while in remission, demonstrating a second complete response and again stopping therapy. Dashed lines represent refractory disease in which continued cytotoxic therapy was delivered without observable benefit. Chemo indicates chemotherapy. (C) An open‐label, phase 2 study to assess the efficacy of 4 approaches to metastatic FPRMS is illustrated. The shared primary endpoint is 3‐year event‐free survival (EFS), with the exception of arm C (see E). ARST0431 indicates the Children's Oncology Group trial “High‐Dose Combination Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Metastatic Rhabdomyosarcoma or Ectomesenchymoma (clinicaltrials.gov identifier NCT00354744); ARST0531, Children's Oncology Group trial “Combination Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Rhabdomyosarcoma” (clinicaltrials.gov identifier NCT00354835); CR, complete response; D9803, Children's Oncology Group trial “Combination Chemotherapy in Treating Patients With Previously Untreated Rhabdomyosarcoma” (clincialtrials.gov identifier NCT00003985); PFS, progression‐free survival; VAC, vincristine, actinomycin‐D, and cyclophosphamide; Vino/C, vinorelbine and oral cyclophosphamide; VinoAC, vinorelbine, actinomycin‐D, and cyclophosphamide. (D) Plasma tumor DNA (ptDNA) and circulating tumor cells (CTCs) will be collected at specified intervals across all arms, with focused collections that may be of interest for a particular arm highlighted in yellow (ie, the rate of decline may be greater with an augmented first strike on arm A; circulating cell‐free ptDNA at a second‐strike transition point that may indicate whether different populations are being eliminated by a second strike in arm B; CTCs with single‐cell RNAseq to evaluate genetic changes over time in arm C with the shared primary endpoint of 3‐year EFS). NED indicates no evidence of disease. (E) A schema for adaptive therapy in metastatic FPRMS is illustrated in which therapy will be withheld when the tumor responds, corresponding with arm C (see C) of an upcoming, evolutionary inspired FPRMS trial. PD indicates progressive disease; PR, partial response; SD, stable disease.
