Abstract
Aim
Caregiver‐child reading is advocated by health organisations, citing cognitive and neurobiological benefits. The influence of home literacy environment (HLE) on brain structure prior to kindergarten has not previously been studied.
Methods
Preschool‐age children completed assessments of language (EVT‐2, CTOPP‐2 Rapid Object Naming) and emergent literacy skills (Get Ready to Read!, The Reading House) followed by diffusion tensor imaging (DTI). Parents completed a survey of HLE (StimQ‐P2 READ), which has four subscales. DTI measures included axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) and fractional anisotropy (FA).
Results
Forty‐seven children completed DTI (54 ± 7 months, range 36‐63; 27 girls). StimQ‐P2 READ scores correlated with higher EVT‐2, GRTR and TRH scores, controlling for age and gender (P < .01), and also with lower AD, RD and MD in tracts supporting language and literacy skills, controlling for age, gender and income (P < .05, family‐wise error corrected). Correlations were strongest for the Bookreading Quantity subscale, including with higher scores on all cognitive measures including CTOPP‐2, and also with higher FA in left‐lateralised literacy‐supporting tracts, controlling for age, gender and income.
Conclusion
More nurturing home reading environment prior to kindergarten may stimulate brain development supporting language and literacy skills, reinforcing the need for further study.
Keywords: brain white matter microstructure, diffusion weighted magnetic resonance imaging, emergent literacy, home reading environment

Abbreviations
- AAP
American Academy of Pediatrics
- CTOPP
comprehensive test of phonological processing
- DTI
diffusion tensor imaging
- EVT
expressive vocabulary test
- FWE
family‐wise error
- GRTR
Get Ready to Read
- MRI
magnetic resonance imaging
- SES
socio‐economic status
- TRH
The Reading House
- WHO
World Health Organization
Key notes.
Benefits of nurturing home literacy environment (HLE) are widely cited, yet associations with structural brain development in young children have not previously been studied.
This study found associations between more stimulating HLE and higher measures of brain white matter integrity related to language and literacy, and also with related cognitive measures.
Programmes and policies encouraging caregiver‐child reading should be encouraged, especially during stages of rapid brain development prior to kindergarten.
1. INTRODUCTION
Emergent literacy is defined as ‘a developmental continuum between pre‐reading and reading (involving) skills, knowledge and attitudes that are precursors to reading and writing'.1 This process begins in infancy and is influenced by family history, medical concerns (eg prematurity, hearing loss), excessive screen time and facets of home literacy environment (HLE).2, 3 HLE involves access to books, and frequency and quality of ‘shared’ reading, typically involving a parent and child.4 Many children arrive at school at a disadvantage in reading readiness, unlikely to catch up with peers as academic demands accelerate.5 Thus, the American Academy of Pediatrics (AAP) recommends literacy promotion during well‐child visits beginning in infancy, citing cognitive, social‐emotional and neurobiological benefits of stimulating shared reading routines.6 The World Health Organization (WHO) recently released recommendations for children under age 5, encouraging reading and storytelling with caregivers as alternatives to screen time to promote child health and development.7
As reading is a relatively recent human invention, there is no hardwired reading network in the brain. Instead, brain networks that evolved for other functions (eg language, vision, attention) are integrated through reading exposure and practice.8 While reading can be acquired at any age, this process is most efficient during the span of maximal brain plasticity in early childhood.9 In addition to synapse‐level effects, constructive experiences fuel organisation and myelination of white matter tracts, which enhance signal conduction within and between these networks,9 summarised by the maxim attributed to neuroscientist Donald Hebb: ‘Neurons that fire together, wire together’. Recent evidence suggests benefits of HLE on brain function in preschool‐age children prior to kindergarten, including access to books, reading frequency, story format and parent‐child verbal interactivity during the story.10, 11, 12 Involved areas include ones supporting language, visual imagery and working memory, particularly in the left hemisphere, yet association with structural brain development at this age has not previously been studied.
Diffusion tensor magnetic resonance imaging (DTI) is a powerful, non‐invasive means to quantify white matter integrity in the brain and its influence by various factors.9 Established DTI parameters are based on the diffusion of water within 3‐dimensional measurement voxels and include axial diffusivity (AD) along the major orientation axis, radial diffusivity (RD) along the perpendicular axes, and mean diffusivity (MD)—the overall magnitude of diffusion.13 A fourth parameter is fractional anisotropy (FA), which is a scalar value between 0 and 1 associated with the degree of restriction or directionality of water diffusion within voxels.13 Together, these parameters estimate microstructural factors including axon diameter, density and orientation, membrane permeability, and myelination.13 While subject to confounding factors such as crossing fibres and non‐linear orientation, both higher FA and concordantly lower AD, RD and MD have been associated with higher language and pre‐literacy abilities in children. The aim of this study was to use DTI to explore the relationship between HLE and these indices of white matter integrity prior to kindergarten, particularly major tracts supporting language and emergent literacy skills: (a) arcuate fasciculus (AF), which connects Wernicke's and Broca's areas via a dorsal route (vocabulary, syntax, complex language),14 (b) inferior longitudinal fasciculus (ILF), which connects Wernicke's and Broca's areas via a ventral route (semantic processing, imagery),15 and (c) uncinate fasciculus (UF), which connects anterior temporal and limbic areas with inferior frontal areas (semantic association, rapid naming and executive functions).16 Our hypothesis was that more stimulating HLE would be associated with greater integrity in these language‐ and literacy‐supporting tracts (ie higher FA and lower AD, RD, MD), particularly in the left hemisphere, and higher scores on cognitive correlate measures, controlling for child age, gender and household income.
2. METHODS
2.1. Participants
A total of 69 parent‐child dyads were recruited via advertisement at an academic medical centre and surrounding paediatric primary care clinics. Inclusion criteria were 3‐5 years old, born at least 36 weeks of gestation, native English‐speaking household, no history of neurodevelopmental disorder conferring risk of language delays, no prior or current kindergarten, and no contraindications to MRI such as claustrophobia or metal implants. Families were compensated for time and travel, and this study was approved by our Institutional Review Board.
2.2. Home literacy environment
Research coordinators administered the StimQ‐P2 READ to a custodial parent in a private room before or during the child's MRI. StimQ‐P2 is a validated parent report measure of home cognitive environment involving children between 36 and 60 months old, involving four subscales.17 For the purposes of this study, only the READ subscale was administered, which captures four domains of shared reading: (a) Quantity, (b) Diversity of concepts, (c) Diversity of content and (d) Quality (interactivity).
2.3. Cognitive assessments and analyses
Four standardised cognitive assessments were administered to each child before MRI: Expressive Vocabulary Test, Second Edition (EVT‐2), Comprehensive Test of Phonological Processing, Second Edition (CTOPP‐2; Rapid Object Naming subtest), Get Ready to Read! (GRTR) and The Reading House (TRH). EVT‐2 is a norm‐referenced assessment of expressive vocabulary for children of age 2.5 years and older. The CTOPP‐2 is a comprehensive, norm‐referenced instrument designed to assess phonological processing abilities as prerequisites to reading fluency. The Rapid Object Naming subtest is designed to assess efficiency of retrieval of information from memory and execution of operations quickly in younger children who have not yet mastered letters or numbers (ie speed of processing). Get Ready to Read! is a norm‐referenced assessment of core emergent literacy skills for children 3‐6 years of age, predictive of reading outcomes.18 The Reading House is a direct assessment of emergent literacy skills in children's book format that was recently validated in children between 3 and 4 years of age.19
Responses for surveys and assessments were entered into a REDCap database for analysis. Spearman‐rho (r ρ) correlation coefficients were first calculated between StimQ‐P2 READ scores and each of the four assessments. Spearman‐rho was chosen given relatively small sample size for behavioural analyses, warranting a conservative, non‐parametric approach. StimQ‐P2 READ scores were then applied as the predictor variable in a series of multiple linear regression models, with CTOPP‐2, EVT‐2, GRTR and TRH scores as the dependent variable, respectively. Child age and gender were applied in each model as covariates, with a threshold of statistical significance of P < .05. Income level was considered as a covariate but excluded, due to moderate collinearity with StimQ‐P2 READ scores (r ρ = .43), which negatively impacted statistical power.
2.4. Diffusion tensor imaging
Details of play‐based acclimatisation techniques used with children prior to MRI are described by Hutton et al11 All children were awake and non‐sedated during MRI. The MRI protocol involved a T1‐weighted anatomical scan lasting approximately 6 minutes, four BOLD fMRI sequences lasting approximately 5 minutes each (resting state and three active tasks), and DTI lasting approximately 8 minutes, each separated by approximately 2 minutes for the child to rest. Children were allowed to watch a video of their choice during DTI.
Magnetic resonance imaging was conducted using a three Tesla Philips Achieva scanner equipped with a 32‐channel head coil. For DTI, sixty‐one diffusion‐weighted (b‐value = 2000 s/mm2) images were acquired using a spin echo‐planar imaging sequence with the following parameters: TR/TE = 5000/88 mseconds; acquisition matrix: 96 × 96; 66 slices; slice thickness = 2mm; 2 × 2 mm in‐plane resolution; in‐plane parallel reduction factor = 1.5; multi‐band factor = 3. Data were visually assessed for quality issues (eg pervasive motion/artefact, inadequate coverage) prior to processing and analyses conducted using the Functional MRI of the Brain (FMRIB) Software Library (FSL), version 5.0.11 (www.fmrib.ox.ac.uk/fsl). Diffusion‐weighted images were corrected for subject movement and other artefacts using FSL's eddy function. Mean and standard deviation framewise movement across subjects was 0.54 ± 0.29 mm, and mean and standard deviation frequency of outlier slices was 1.3 ± 1.1%. Slices identified as outliers were replaced with predictions made by the Gaussian Process (eddy's ‘—repol’ option with default parameters except single slice and groupwise outlier detection for multi‐band acquisition). The eddy‐corrected diffusion‐weighted images and rotated b‐vectors were used to fit a standard diffusion tensor model at each voxel using FSL's dtifit function. Maps of fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD) and radial diffusivity (RD) were calculated from the principle eigenvalues of the diffusion tensor using standard methods.
2.5. Tract‐based spatial statistics
We performed two analyses using FSL's tract‐based spatial statistics pipeline to test for associations between DTI measures and StimQ‐P2 READ: one ‘whole‐brain’ analysis and one hypothesis‐driven, tract‐specific analysis. First, individual FA maps were nonlinearly warped into alignment with the FMRIB58_FA template and the same warp fields were applied to each diffusivity map (AD, RD, MD) to bring them into alignment with standard space. For the whole‐brain analysis, an analysis mask was created by skeletonising the group mean FA map after thresholding at a value of 0.2. For the second analysis, a new analysis mask was created by intersecting the whole‐brain mask with binarised probability maps of the left arcuate fasciculus (AF), inferior longitudinal fasciculus (ILF) and uncinate fasciculus (UF), three tracts clearly associated with language and literacy abilities on children and assessed via the selected cognitive measures (language, emergent literacy, rapid naming).16, 20, 21 Left‐sided tracts were selected given well‐described structural and functional lateralisation supporting these abilities at this age.22, 23 A general linear model approach was used to test for correlations between StimQ‐P2 READ scores and each of the four DTI measures at each voxel, with StimQ‐P2 READ scores, de‐meaned age, de‐meaned gender and de‐meaned household income comprising the design matrix. FSL's randomise function was used to perform 10 000 random permutations of this multiple regression analysis, and family‐wise error rate was controlled by using threshold‐free cluster enhancement.24 Significant clusters in the whole‐brain analysis were cross‐referenced to known white matter tracts using a white matter tractography atlas created by Rojkova et al25 (Table 2).
Table 2.
Average association between diffusivity measures and StimQ‐P2 READ scores in left and right hemispheric fibre tracts, particularly supporting higher‐order association, controlling for effects of age, gender and income
| White matter tract | Regions connected | Major functions | AD | MD | RD | |||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |||
| Arcuate fasciculus—anterior |
Inferior frontal Caudal temporal Inferior parietal (Broca's‐Wernicke's) |
Language | −8.1 | −8.6 | −5.4 | −5.9 | −4.0 | −4.5 |
| Arcuate fasciculus—long | −8.8 | −8.4 | −6.0 | −5.9 | −4.6 | −4.6 | ||
| Arcuate fasciculus—posterior | −10.2 | −9.6 | −7.0 | −7.0 | −5.4 | −5.6 | ||
| Cingulum | Frontal, parietal, medial temporal, subcortical | Emotion, learning, memory | −9.0 | −9.9 | −5.9 | −5.8 | −4.6 | −3.9 |
| Corpus callosum | Left‐right cerebral hemispheres | Communication between hemispheres | −8.7 | −8.8 | −5.8 | −5.9 | −4.4 | −4.6 |
| Frontal commissural | Frontal‐frontal | Executive | −7.3 | −8.4 | −5.0 | −5.4 | −4.0 | −4.1 |
| Inferior fronto‐occipital fasciculus | Frontal‐occipital/temporal | Association, language | −8.6 | −8.9 | −5.8 | −5.9 | −4.4 | −4.4 |
| Inferior longitudinal fasciculus | Occipital‐temporal | Visual association/imagery, language | −10.7 | −9.3 | −6.4 | −6.0 | −4.3 | −4.4 |
| Pons | Forebrain‐hindbrain (cerebellum) | Arousal, relay sensory/learning information | −7.6 | −5.6 | −4.7 | −3.2 | −3.3 | −2.1 |
| Superior longitudinal fasciculus III (ventral) | Supramarginal‐ventral prefrontal | Somatosensory, expressive language, working memory | −7.1 | −8.0 | −4.8 | −5.6 | −3.6 | −4.4 |
| Superior longitudinal fasciculus II (major) | Inferior parietal‐dorsolateral prefrontal | Visual‐spatial attention/association | −8.4 | −8.9 | −5.7 | −6.3 | −4.3 | −4.9 |
| Superior longitudinal fasciculus I (dorsal) | Superior parietal‐dorsal/medial prefrontal, supplementary motor | Motor task selection/association | −8.0 | −8.6 | −6.0 | −6.2 | −5.0 | −5.1 |
| Uncinate fasciculus | Orbitofrontal‐anterior temporal | Limbic association, language (rapid naming) | −3.6 | −5.2 | −2.3 | −3.4 | −1.6 | −2.5 |
Values are in units of 10‐6mm2s‐1 per point of StimQ‐P2 READ. Tracts with significant clusters (see Figure 2) spanning greater than 10% of their volume are in bold.
3. RESULTS
3.1. Demographics
Of 69 children, 47 completed DTI (age: 54.3 ± 7.5 months, range 37‐63 months; 20 boys, 27 girls). Demographics are summarised in Table 1. There was no significant difference in gender, race or household income between children completing DTI and those who did not. Mean age of children completing DTI was significantly older (mean 54 ± 8 months, range 37‐63 completing; vs 46 ± 6 months, 36‐57), and maternal education was significantly higher (most college graduates completing, vs most with some college who did not).
Table 1.
Subject demographics (n = 47)
| N (%) | N | Mean ± SD | (Min, Max) | |
|---|---|---|---|---|
| Child age (years) | ||||
| 3‐3.9 | 10 (21) | |||
| 4‐4.9 | 22 (47) | |||
| 5‐up | 15 (32) | |||
| Child gender | ||||
| Male | 20 (43) | |||
| Female | 27 (57) | |||
| Child race | ||||
| African American/black | 15 (32) | |||
| Caucasian/white | 31 (66) | |||
| Other | 1 (2) | |||
| Parental marital status | ||||
| Single | 12 (26) | |||
| Married | 34 (72) | |||
| Divorced/separated | 1 (2) | |||
| Annual household income ($) | ||||
| ≤25 000 | 8 (17) | |||
| 25 001‐50 000 | 6 (13) | |||
| 50 001‐100 000 | 13 (28) | |||
| 100 001‐150 000 | 11 (23) | |||
| Above 150 000 | 9 (19) | |||
| Maternal education | ||||
| High school or less | 3 (7) | |||
| Some college/associate | 7 (15) | |||
| College graduate | 19 (40) | |||
| More than college | 18 (38) | |||
| StimQ‐P2 READ total | 47 | 14.7 ± 2.9 | (5, 18) | |
| Bookreading quantity | 47 | 7.1 ± 2.1 | (2, 9) | |
| CTOPP‐2 rapid object naming scaled | 40 | 9.4 ± 3.3 | (2, 15) | |
| EVT‐2 scaled | 46 | 113.1 ± 15.6 | (88, 144) | |
| GRTR total | 47 | 9.6 ± 5.0 | (1, 22) | |
| TRH total | 47 | 13.8 ± 4.7 | (2, 19) | |
3.2. Cognitive‐environmental assessments
Scores for parental HLE assessment (StimQ‐P2 READ) and child cognitive assessments are summarised in Table 1. Altogether, 64% of children scored within the average vocabulary range on the EVT‐2.
There were significant positive correlations between higher StimQ‐P2 READ scores and higher EVT‐2 (r ρ = .45), GRTR (r ρ = .34) and TRH (r ρ = .34) scores (P < .01). These relationships remained significant controlling for child age and gender (P < .01). StimQ‐P2 READ scores were not significantly correlated with CTOPP‐2 scores. Scatter plots illustrating significant relationships are shown in Figure 1.
Figure 1.
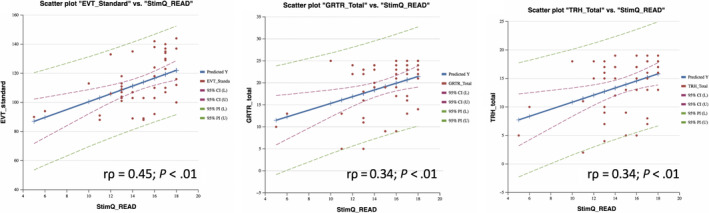
Scatter plots of StimQ‐P2 READ total scores vs language and literacy measures. Scatter plots of StimQ‐P2 READ total scores vs expressive vocabulary (EVT‐2 scaled) and emergent literacy (The Reading House and Get Ready to Read!) scores. Solid line: least squares fit, dashed green: 95% confidence bounds of slope, dashed red: 95% prediction interval for new observations
In terms of subdomain scores, correlations were strongest involving Bookreading Quantity for all outcomes: EVT‐2 (r ρ = .48), GRTR (r ρ = .35), TRH (r ρ = .40) and also significant for CTOPP‐2 (r ρ = .48; all P < .01). Relationships for each remained significant in regression models controlling for age and gender (P < .01). Correlations were not significant involving Diversity of Bookreading Concepts, Content or Bookreading Quality.
3.3. Diffusion tensor imaging
Higher StimQ‐P2 READ scores were correlated with significantly lower AD (Nvoxels = 11 508; t‐stat = 2.40 ± 0.54), RD (Nvoxels = 1608; t‐stat = 2.89 ± 0.44) and MD (Nvoxels = 15 060; t‐stat = 2.62 ± 0.48), controlling for child age, gender and household income (P < .05, family‐wise error [FEW]‐corrected, 2‐tailed test). Figure 2 illustrates the extent and degree of these correlations in whole‐brain analyses, with parameter estimates from multiple regression analyses showing the adjusted change in AD, MD and RD per unit increase in StimQ‐P2 READ score. These relationships were most extensive involving AD and MD, particularly involving the AF, cingulum, inferior fronto‐occipital fasciculus (IFOF), ILF, corpus callosum and portions of the superior longitudinal fasciculus (SLF), as described in Table 2. Significant association between StimQ‐P2 READ scores and FA was not found.
Figure 2.
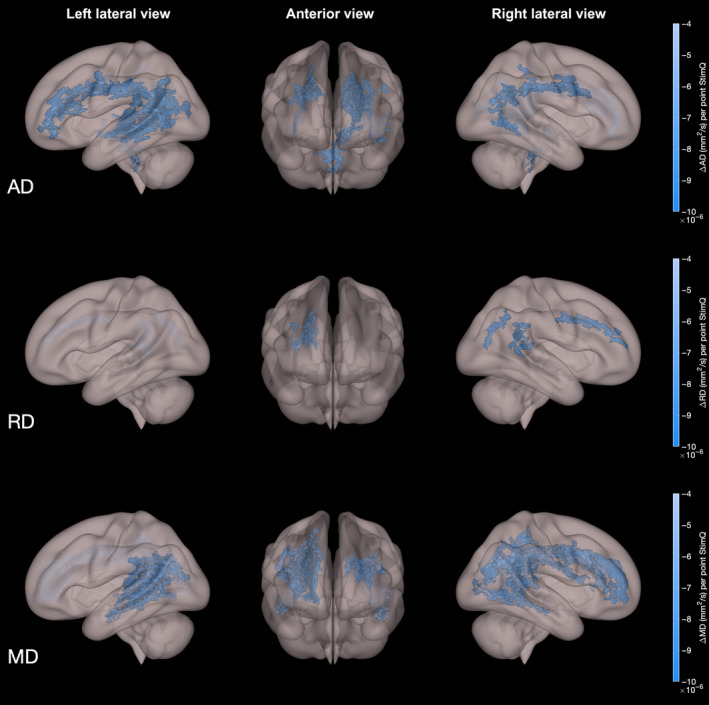
Diffusion tensor imaging parameter maps (AD, RD, MD) from whole‐brain analyses involving associations with StimQ‐P2 READ scores. White matter voxels exhibiting significant association between home literacy environment and decreased axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) in whole‐brain analysis, controlling for age and household income (P < .05, FWE‐corrected). Colour indicates the magnitude of association (ie the change in the DTI parameter for every point increase in StimQ‐P2 READ score), controlling for child age, gender and household income level (P < .05, FWE‐corrected)
In the hypothesis‐driven analysis involving tracts in the left hemisphere, StimQ‐P2 READ scores were associated with significantly lower AD (Nvoxels = 4996; t‐stat = 2.31 ± 0.63), RD (Nvoxels = 2831; t‐stat = 2.41 ± 0.53) and MD (Nvoxels = 5802; t‐stat = 2.49 ± 0.55) localised to the AF and a lesser extent of the ILF, controlling for age, gender and income (P < .05, FWE corrected, 2‐tailed test). These relationships are shown in Figure 3. Significant association between StimQ‐P2 READ scores and FA was not found.
Figure 3.
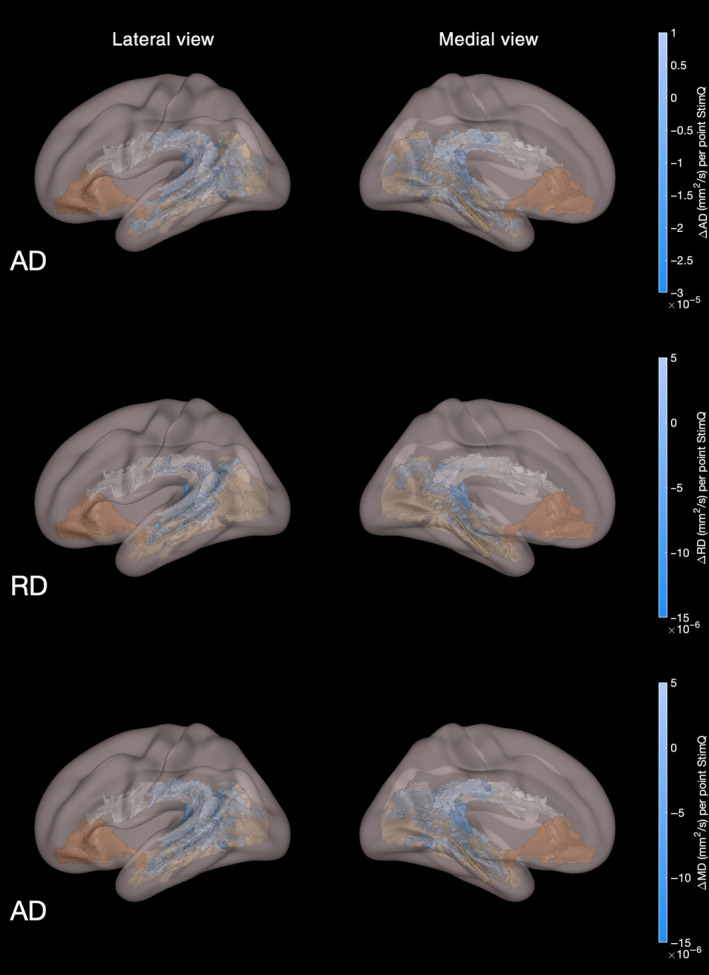
Diffusion tensor imaging parameter maps (AD, RD, MD) from hypothesis‐based analyses involving associations with StimQ‐P2 READ scores. White matter voxels exhibiting significant association between home literacy environment and decreased axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) involving three white matter tracts in the left hemisphere supporting language and literacy skills: arcuate fasciculus (white), inferior longitudinal fasciculus (tan), uncinate fasciculus (brown). Colour indicates the magnitude of association (ie the change in the DTI parameter for every point increase in StimQ‐P2 READ score), controlling for child age, gender and household income level (P < .05, FWE‐corrected)
Higher StimQ‐P2 READ Bookreading Quantity scores were correlated with significantly higher FA in both whole‐brain (Nvoxels = 5344; t‐stat = 2.17 ± 0.49) and hypothesis‐driven (Nvoxels = 2404; t‐stat = 2.04 ± 0.60) analyses, and lower RD in hypothesis‐driven (Nvoxels = 124; t‐stat = 3.22 ± 0.52) analyses, controlling for child age, gender and household income (P < .05, family‐wise error [FEW]‐corrected, 2‐tailed test). These were most extensive involving FA, particularly involving corpus callosum and left‐lateralised tracts including AF, ILF and UF, as shown in Figure 4.
Figure 4.
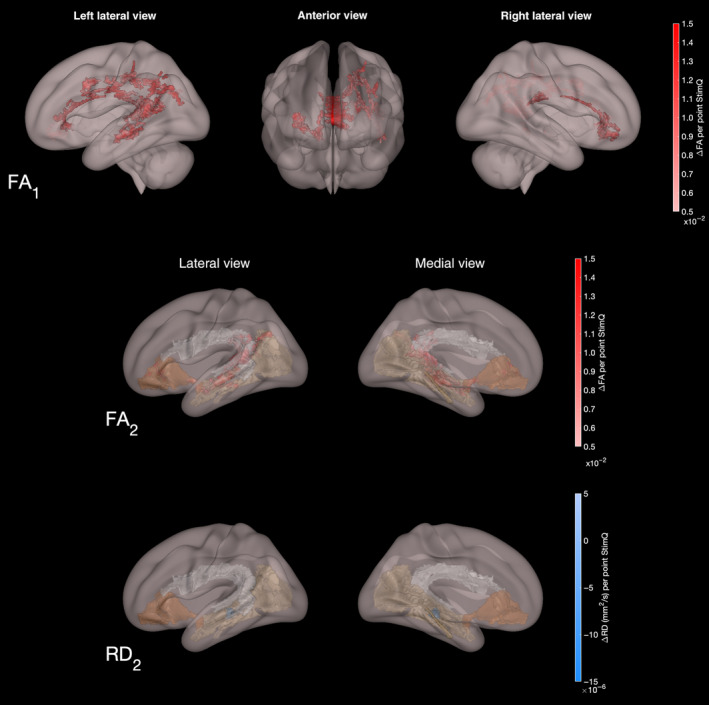
Diffusion tensor imaging parameter maps (FA, RD) from whole‐brain and hypothesis‐based analyses involving associations with StimQ‐P2 READ Bookreading Quantity subdomain scores. White matter voxels exhibiting significant association between Bookreading Quantity subdomain scores and increased fractional anisotropy and decreased radial diffusivity in whole‐brain (FA1) and hypothesis‐based (FA2, RD2) analyses, controlling for age, gender and household income (P < .05, FWE‐corrected). Colour indicates the magnitude of association (ie the change in the DTI parameter for every point increase in StimQ‐P2 READ score), controlling for child age and household income level (P < .05, FWE‐corrected)
4. DISCUSSION
Literacy is a major social determinant of health, its neurobiological infrastructure shaped during early childhood.26 Shared reading and the HLE in which it occurs are catalysts for this emergent process, advocated by the AAP, WHO, and numerous agencies and reading advocacy groups.6, 7 The aim of this study was to explore structural neurobiological correlates of HLE prior to kindergarten, a critical span of neural growth and plasticity.9 Given privileged access during clinic visits when families are receptive, paediatricians (alongside childcare professionals and early childhood educators) are uniquely positioned to convey empowering, informed guidance reinforcing the importance of nurturing shared reading routines on cognitive, social‐emotional and neurobiological health.6
This study is the first to our knowledge to document associations between HLE and measures of brain white matter microstructure supporting language and emergent literacy skills in preschool‐age children, controlling for age and household income. Maturation of white matter involves changes in axonal density and myelination, which are responsive to environmental experiences.9 In terms of DTI parameters, AD is thought to largely reflect organisation of axons in parallel bundles, RD axonal myelination, and MD and FA, aspects of both.13 While AD can be challenging to interpret due to non‐linear architecture,27 decreases in RD and MD, and increases in FA, are consistently observed with increasing age and in response to environmental stimulation, and considered reliable indicators of healthy brain development.28 While associations between more stimulating HLE and lower AD, MD and RD found in this study align with this evidence and our hypothesis, significant association was not found between the composite StimQ‐P2 READ measure of HLE and FA, though this was found for the Bookreading Quantity subdomain. While these findings seem inconsistent, a reasonable interpretation involves simultaneous, proportionate decreases in AD and RD with consequently minimal change in FA (a scalar value dependent on these parameters) related to aggregate HLE, and less proportionate differences related to quantitative aspects, such as relatively greater organisation. Previous studies have found concordant decreases in AD, RD and MD associated with higher language and literacy abilities in children29 as found here in both whole‐brain and hypothesis‐driven analyses, and also with higher FA,21, 30 reinforcing this interpretation.
Structural markers for language performance involving DTI parameters (particularly FA and RD) have been described as young as 3 years old,31 in both full‐term and preterm children.29 The arcuate fasciculus (AF), which connects receptive (Wernicke's) and expressive (Broca's) language brain areas,20 is most strongly associated with language and emergent literacy, including phonological processing, syntax and vocabulary.30 The ILF, which connects temporal‐occipital and anterior temporal areas, is also associated with language and literacy skills, particularly semantic processing and visual imagery.15, 21 Recent functional MRI studies involving a story listening task documented higher activation in left‐sided brain areas connected via the AF and/or ILF for preschool‐age children exposed to both more stimulating HLE and higher maternal shared reading quality.10, 11 Thus, our finding of higher HLE associated with lower AD, RD and MD in the AF and ILF in the context of higher EVT‐2 scores suggests more developed infrastructure for language in children exposed to more reading at home. While muted in the whole‐brain analysis, associations with these core language tracts in the left hemisphere are evident in the hypothesis‐based analysis, attributable to increased statistical power. This relationship with HLE is not surprising given the protracted maturation of frontal‐temporal tracts such as AF and ILF,20 rendering them sensitive to home‐environmental routines such as talking, singing and reading.6 Association between the Bookreading Quantity scores, which incorporate routines, and higher FA and lower RD in strongly left‐lateralised language tracts reinforces their potential importance at this age.
Interestingly, negative associations found between DTI parameters (AD, MD, RD) and HLE in whole‐brain analyses for the composite HLE measure were largely bilateral (some right‐lateralised), consistent with the reliance of language and emergent literacy abilities on a distributed, bilateral network that becomes more specialised with age.23, 31 The involvement of the right hemisphere in language and literacy has been well‐documented in children, including the IFOF, ILF, UF and SLF.32 While speculative, associations found in this study may also reflect broader benefits of nurturing HLE described in behavioural studies, such as attention, working memory, imagination and emotional control.33 For example, microstructural integrity of the cingulum and SLF has been associated with working memory and attention in children, each extensively associated with HLE in this study.34 Future studies involving correlate measures of these abilities would be useful to elucidate these potential benefits in terms of brain structure and function.
This study did not find significant correlation between the composite HLE measure and phonological/naming skills (CTOPP‐2), which aligns with previous research failing to consistently document such effects in young children.3 However, correlation with Bookreading Quantity scores was robust, suggesting more nuanced relationships. Rapid automatised naming (RAN) is a critical emergent literacy skill supporting narrative comprehension and reading fluency that is often implicated in reading difficulties.35 Interestingly, significant positive correlation was also found between Bookreading Quantity and higher FA in the UF, which is thought to support RAN abilities,16 but not with the composite HLE measure. It is possible that in addition to reading routines, RAN may be dependent on a variety of factors such as genetic capacity, other environmental influences and/or relatively protracted maturation of the frontal lobe,9 topics worthy of future study.
While children in this study were pre‐kindergarten and not yet reading independently, findings involve tracts supporting foundational emergent literacy skills including language, executive functions (eg working memory), multi‐modal association (eg words‐emotion) and visual imagery (Table 2). Emergent literacy development has been associated with increases in FA and decreases in RD and MD in the AF,30 while lower FA and higher MD in the AF, ILF and SLF have been associated with lower emergent skills.36, 37 Decreased AD, MD and RD in cerebellar, temporal, superior frontal and parietal white matter (eg AF, pons, ILF) have also been associated with reading development and shown to predict reading outcomes,21 with increases in myelination, axonal densities and/or fibre coherence suggested as proposed mechanisms.38 Thus, extensive associations between higher composite HLE and lower AD, MD, and RD in the AF, ILF and other tracts involved with emergent literacy skills, in the context of higher EVT‐2, GRTR and TRH scores, suggest more developed neural infrastructure supporting emergent literacy in children exposed to more books and reading at home prior to kindergarten. Similarly, positive associations between Bookreading Quantity and higher FA in left‐lateralised tracts known to support literacy development reinforce the importance of consistent reading routines to catalyse this process.
These findings also align with a recent MRI study involving 8‐12‐year‐old children that found higher functional connectivity within the emerging ‘reading network’ correlated with more time spent reading, and the opposite for screen time.39 Displacement effects of screen time on home reading practices have been documented in behavioural studies,40 and associations between more screen time and lower measures of white matter integrity were recently described in a related DTI study involving preschool‐age children.41 It seems reasonable to speculate that at least some of the adverse effects of excessive screen time in young children may be due to less nurturing, protective shared reading time. Longitudinal studies involving a range of reading and health outcomes are critical to discern long‐term relationships and effects.
This study has limitations that should be noted. While representing a range of income and educational backgrounds, the sample involved largely families of higher‐SES, yet revealed results consistent with current literature regarding benefits of HLE, and DTI analyses controlled for household income. We suspect that these findings may have been even more pronounced in families of lower‐SES, where disparities in language, pre‐literacy and other cognitive abilities are well‐documented.42 Larger studies are needed to explore the extent to which home reading practices mediate these relationships, as well as those between SES and early brain development. Due to moderate collinearity between StimQ‐P2 READ scores and income, and relatively small sample size, correlations in the behavioural analyses did not reach significance controlling for this covariate, yet did for the Bookreading Quantity subdomain, suggesting outsize influence of this routine‐focused aspect of HLE. StimQ‐P2 READ is a parent report measure subject to social desirability bias, yet is established in paediatric literature.17 While DTI is a powerful method to study brain white matter, it is vulnerable to artefacts attributable to non‐uniform directionality of fibre tracts, such as crossing fibres.13 Thus, DTI is highly sensitive but often non‐specific, unable to distinguish cellular‐level properties such as axon coherence or fibre crossings.13 However, analyses involved well‐defined tracts and findings were concordant in terms of three established DTI parameters, which aligns with previous research involving language and literacy skills in children. Significant relationship was not found between the composite measure of HLE and FA, an oft‐cited estimate of white matter integrity, yet the pattern of results (lower AD, RD, MD; no difference in FA) has been described in young children,29 and associations between the Bookreading Quantity subdomain and FA were found in tracts supporting language and literacy skills. Diffusion‐weighted images were acquired at the end of a long MRI session, resulting in a lower than usual success rate (68%), possibly conferring bias towards older children with higher attention skills. However, DTI is a task‐free paradigm not influenced by performance factors, a range of children completed the session, and our hypothesis‐driven analysis was not attention‐related. Most importantly, while this study found compelling associations between HLE and both cognitive and neurobiological measures, its cross‐sectional design cannot discern causation or directionality of effects, which requires a longitudinal approach. Similarly, modest size limits the number of covariates and types of analyses that would be possible with a larger sample.
This study also has important strengths. Its sample size of 47 preschool‐age children is remarkable given the difficulty of successfully conducting MRI at this age. StimQ‐P2 READ is a validated measure providing a holistic view of HLE, and also specific behaviours such as routines. The DTI analyses accounted for subject motion, survived stringent correction for multiple comparisons (FWE) and controlled for potential confounders of age and income. Findings included characterisation of associations with HLE via whole‐brain and hypothesis‐driven analyses, the latter with increased statistical power in the context of extensive behavioural2, 3 and recent fMRI evidence10, 11 involving preschool‐age children. Four validated assessments, including three directly administered to the child, provided clear cognitive‐behavioural correlates for the DTI findings. In terms of practical application, this study provides a novel, neurobiological lens to view recommendations regarding the importance of consistent shared reading routines6, 7 and programmes designed to enhance HLE, such as Reach Out and Read. Longitudinal studies are needed to explore whether associations observed here reflect longer‐term benefits, notably for kindergarten readiness and ultimate reading abilities, to ensure that each child's story is happy, healthy and productive.
5. CONCLUSIONS
In this study involving 47 preschool‐age children, more stimulating home literacy environment (HLE) was associated with greater microstructural integrity (organisation and myelination) of white matter tracts supporting language and emergent literacy skills and with corresponding cognitive‐behavioural measures. These findings represent novel neurobiological evidence for potential benefits of HLE and programmes that encourage nurturing shared reading routines beginning in infancy, and suggest a need for further study.
CONFLICTS OF INTEREST
Dr Hutton is the author of The Reading House screening measure used in this study, which is licensed by Cincinnati Children's Hospital, with royalties determined via customary policies. Dr Hutton serves on the Medical Advisory Board of Reach Out and Read, with no financial compensation. Tom DeWitt is the Chairman of national Reach Out and Read with no financial compensation. The remaining authors have no potential conflicts of interest to disclose.
FUNDING INFORMATION
This study was funded by a Procter Scholar Award from the Cincinnati Children's Research Foundation (Hutton).
INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
ACKNOWLEDGEMENTS
The authors would like to thank research coordinators Arielle Wilson and Amy Kerr for overseeing data collection, data entry and quality control. They also thank the Cincinnati Children Hospital Research Foundation for their generous support of early‐career investigators and this work via a Procter Scholar Award.
Hutton JS, Dudley J, Horowitz‐Kraus T, DeWitt T, Holland SK. Associations between home literacy environment, brain white matter integrity and cognitive abilities in preschool‐age children. Acta Paediatr. 2020;109:1376–1386. 10.1111/apa.15124
REFERENCES
- 1. Whitehurst GJ, Lonigan CJ. Child development and emergent literacy. Child Dev. 1998;69(3):848‐872. [PubMed] [Google Scholar]
- 2. Hamilton LG, Hayiou‐Thomas ME, Hulme C, Snowling MJ. The home literacy environment as a predictor of the early literacy development of children at family‐risk of dyslexia. Sci Stud Read. 2016;20(5):401‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Early Literacy Panel . Developing early literacy: report of the national early literacy panel. Washington, DC: National Institute for Literacy; 2008. [Google Scholar]
- 4. Bus A, van Ijzendoorn M, Pellegrini A. Joint book reading makes for success in learning to read: a meta‐analysis on intergenerational transmission of literacy. Rev Educ Res. 1995;65(1):1‐21. [Google Scholar]
- 5. Center on Children and Families at Brookings . Starting School at a disadvantage: the school readiness of poor children. Washington, DC: Brookings Institution; 2012. [Google Scholar]
- 6. AAP Council on Early Childhood . Literacy promotion: an essential component of primary care pediatric practice. Pediatrics. 2014;134(2):404‐409. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization . WHO guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 8. Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat Rev Neurosci. 2015;16(4):234‐244. [DOI] [PubMed] [Google Scholar]
- 9. Lebel C, Deoni S. The development of brain white matter microstructure. NeuroImage. 2018;182:207‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutton JS, Phelan K, Horowitz‐Kraus T, et al. Shared reading quality and brain activation during story listening in preschool‐age children. J Pediatr. 2017;191:204–211.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hutton J, Horowitz‐Kraus T, Mendelsohn A, DeWitt T, Holland S. Home reading environment and brain activation in preschool children listening to stories. Pediatrics. 2015;136(3):466‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutton JS, Dudley J, Horowitz‐Kraus T, DeWitt T, Holland SK. Functional connectivity of attention, visual, and language networks during audio, illustrated, and animated stories in preschool‐age children. Brain Connect. 2019;9(7):580‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front Neurosci. 2013;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friederici AD. Evolution of the neural language network. Psychon Bull Rev. 2017;24(1):41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papagno C. Naming and the role of the uncinate fasciculus in language function. Curr Neurol Neurosci Rep. 2011;11(6):553‐559. [DOI] [PubMed] [Google Scholar]
- 17. Bellevue Project for Early Language Literacy and Education Success . STIMQ home cognitive environment. 2018; http://pediatrics.med.nyu.edu/developmental/research/the-belle-project/stimq-cognitive-home-environment. Accessed August, 2019.
- 18. Lonigan C, Wilson S.Report on the revised get ready to read! Screening tool: psychometrics and normative information national center on learning disabilities July 2, 2008. 2008.
- 19. Hutton JS, Justice L, Huang G, Kerr A, DeWitt T, Ittenbach RF. The reading house: a children's book for emergent literacy screening during well‐child visits. Pediatrics. 2019;143(6):20183843 10.1542/peds.2018-3843 [DOI] [PubMed] [Google Scholar]
- 20. Friederici AD. White‐matter pathways for speech and language processing. Handb Clin Neurol. 2015;129:177‐186. [DOI] [PubMed] [Google Scholar]
- 21. Borchers LR, Bruckert L, Dodson CK, et al. Microstructural properties of white matter pathways in relation to subsequent reading abilities in children: a longitudinal analysis. Brain Struct Funct. 2019;224(2):891‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30(11):3563‐3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holland SK, Vannest J, Mecoli M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46(9):533‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83‐98. [DOI] [PubMed] [Google Scholar]
- 25. Rojkova K, Volle E, Urbanski M, Humbert F, Dell'Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct. 2016;221(3):1751‐1766. [DOI] [PubMed] [Google Scholar]
- 26. Horowitz‐Kraus T, Hutton JS. From emergent literacy to reading: how learning to read changes a child's brain. Acta Paediatrica. 2015;104(7):648‐656. [DOI] [PubMed] [Google Scholar]
- 27. Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes‐what do we know? Front Neurol. 2018;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynolds JE, Grohs MN, Dewey D, Lebel C. Global and regional white matter development in early childhood. Neuroimage. 2019;96:49‐58. [DOI] [PubMed] [Google Scholar]
- 29. Dodson CK, Travis KE, Borchers LR, Marchman VA, Ben‐Shachar M, Feldman HM. White matter properties associated with pre‐reading skills in 6‐year‐old children born preterm and at term. Dev Med Child Neurol. 2018;60(7):695‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 2014;24(4):989‐995. [DOI] [PubMed] [Google Scholar]
- 31. Walton M, Dewey D, Lebel C. Brain white matter structure and language ability in preschool‐aged children. Brain Lang. 2018;176:19‐25. [DOI] [PubMed] [Google Scholar]
- 32. Horowitz‐Kraus T, Grainger M, DiFrancesco M, Vannest J, Holland SK. Right is not always wrong: DTI and fMRI evidence for the reliance of reading comprehension on language‐comprehension networks in the right hemisphere. Brain Imaging Behav. 2015;9(1):19‐31. [DOI] [PubMed] [Google Scholar]
- 33. Mendelsohn AL, Cates CB, Weisleder A, et al. Reading aloud, play, and social‐emotional development. Pediatrics. 2018;141(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neurosci Lett. 2010;477(2):72‐76. [DOI] [PubMed] [Google Scholar]
- 35. Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu Rev Psychol. 2012;63:427‐452. [DOI] [PubMed] [Google Scholar]
- 36. Saygin ZM, Norton ES, Osher DE, et al. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early‐reading kindergarten children. J Neurosci. 2013;33(33):13251‐13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeatman JD, Dougherty RF, Rykhlevskaia E, et al. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci. 2011;23(11):3304‐3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Travis KE, Adams JN, Kovachy VN, Ben‐Shachar M, Feldman HM. White matter properties differ in 6‐year old readers and pre‐readers. Brain Structure Function. 2017;222(4):1685‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horowitz‐Kraus T, Hutton JS. Brain connectivity in children is increased by the time they spend reading books and decreased by the length of exposure to screen‐based media. Acta paediatrica. 2018;107:685‐693. [DOI] [PubMed] [Google Scholar]
- 40. Choi JH, Mendelsohn AL, Weisleder A, et al. Real‐world usage of educational media does not promote parent‐child cognitive stimulation activities. Acad Pediatr. 2018;18(2):172‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hutton JS, Dudley J, Horowitz‐Kraus T, DeWitt T, Holland SK. Associations between screen‐based media use and brain white matter integrity in preschool‐aged children. JAMA Pediat. 2019. 10.1001/jamapediatrics.2019.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larson K, Russ SA, Nelson BB, Olson LM, Halfon N. Cognitive ability at kindergarten entry and socioeconomic status. Pediatrics. 2015;135(2):e440‐e448. [DOI] [PubMed] [Google Scholar]


