Summary
Plant survival depends on vascular tissues, which originate in a self‐organizing manner as strands of cells co‐directionally transporting the plant hormone auxin. The latter phenomenon (also known as auxin canalization) is classically hypothesized to be regulated by auxin itself via the effect of this hormone on the polarity of its own intercellular transport. Correlative observations supported this concept, but molecular insights remain limited.
In the current study, we established an experimental system based on the model Arabidopsis thaliana, which exhibits auxin transport channels and formation of vasculature strands in response to local auxin application.
Our methodology permits the genetic analysis of auxin canalization under controllable experimental conditions. By utilizing this opportunity, we confirmed the dependence of auxin canalization on a PIN‐dependent auxin transport and nuclear, TIR1/AFB‐mediated auxin signaling. We also show that leaf venation and auxin‐mediated PIN repolarization in the root require TIR1/AFB signaling.
Further studies based on this experimental system are likely to yield better understanding of the mechanisms underlying auxin transport polarization in other developmental contexts.
Keywords: Arabidopsis thaliana, auxin, auxin canalization, cell polarity, PIN1, TIR1/AFB
Introduction
Plants possess superb abilities to adapt their development to the changing environment. One of them is their capacity to form organized vasculature, which occurs under normal (e.g. leaf venation or when nascent organs connect to the pre‐existing vascular network) and traumatic (e.g. re‐connection of broken vascular strands after wounding) conditions. The latter example occurs frequently within the ontogeny of higher plants (due to grazing or other types of mechanical stress) and is therefore paramount to their survival. This developmentally fascinating process of vasculature formation involves not only (de)differentiation of multiple cell types, but also coordinated cell polarization ultimately leading to the directional transport of compounds through cellular strands (channels).
It has been proposed that vascular strand formation is regulated by auxin via a putative feedback interaction between its cellular perception and intercellular polar transport (Sachs, 1975, 1981; Uggla et al., 1996, 1998; Tuominen et al., 2000; Sauer et al., 2006; Robert et al., 2013). It is known that vasculature formation is indeed spatially associated with the activation of TIR1/AFB signaling (Lavy et al., 2016) and accumulation of polarly distributed PIN‐FORMED (PIN) auxin efflux proteins (Adamowski & Friml, 2015) in the co‐directionally polarized strands of vascular progenitors (auxin canalization; Sauer et al, 2006; Zhang et al., 2010; Balla et al., 2011; Mazur et al., 2016; Prat et al., 2018). Observations of a similar correlation between auxin signaling and auxin transport polarization have also been made during embryonic apical–basal axis establishment (Robert et al., 2013), shoot and root organogenesis (Benkova et al., 2003; Heisler et al., 2005; Bhatia et al., 2016) as well as unexpected process such as the termination of shoot gravitropic response (Rakusova et al., 2016).
The classical, ‘gold standard’ cell biological studies on auxin canalization were based on local auxin application onto the tissues of different plant species (Raven, 1975; Sachs, 1975, 1981), including pea (Pisum sativum) stems (Sauer et al., 2006; Balla et al., 2011). In this setup, auxin‐transporting channels (and subsequently vascular strands) developed from the application site and connected it to the pre‐existing vasculature of a plant. While these observations indicated that auxin canalization occurs via self‐organization rather than pre‐patterning, further implementation of the classical methodology has been hampered by the difficulty of transgenesis in the corresponding plant species.
Previous reports (Berleth et al., 2000; Dettmer et al., 2009; Bennett et al., 2014) suggested that auxin canalization also underlies physiological processes such as vasculature regeneration after wounding (Sauer et al., 2006; Mazur et al., 2016), leaf venation (Scarpella et al., 2006; Cano‐Delgado et al., 2010; Sawchuk & Scarpella, 2013) and auxin‐mediated PIN lateralization in the root (Prat et al., 2018). In particular, it has been shown that leaf vein specification is the result of directional auxin transport mediated by polarized PIN expression demarcating the position of future vascular patterning. From primary broader PIN1 expression domains, the narrow PIN1‐marked routes of auxin transport emerged as polarized groups of cells differentiating into vascular connections in leaves (Scarpella et al., 2006; Wenzel et al., 2007). Thus, studies utilizing the classical experimental model based on local auxin application are likely to yield knowledge not only on auxin canalization in the context of its exogenous application but also in other, more physiological roles.
Which components of auxin perception are involved in its feedback on auxin transport has not been rigorously addressed, but the well‐characterized signaling pathway involving TRANSPORT INHIBITOR RESPONSE1 (TIR1)/AUXIN SIGNALING F‐BOX (AFB) proteins as auxin receptors and the downstream Aux/IAA and auxin response factor (ARF) transcriptional regulators are likely to be implicated (Dharmasiri & Estelle, 2004; Hayashi et al, 2012). Although the molecular mechanisms are not entirely clear, it was shown that downstream processes in leaf vascular patterning are controlled by the auxin response transcription factor MONOPTEROS (MP) through an auxin response element in the AtHB8 gene promoter. AtHB8 seems to be required to constrict cell fate acquisition to gradually narrower areas, leading to the establishment of procambial cell identity during vein development (Donner et al., 2009). Nonetheless, a demonstration that TIR1/AFB nuclear auxin signaling is required for the auxin feedback on auxin transport polarization during canalization, thus regulating processes such as leaf venation and wounding‐induced vasculature regeneration, has not been provided.
Here, we established an experimental system, in which auxin canalization and vasculature formation can be induced by local auxin application. This makes the setup more direct and controllable compared to our previous approach, which involved vasculature regeneration around the wound (Mazur et al., 2016). We use this system in conjunction with genetic, pharmacological and cell biological methods to demonstrate the requirement of TIR1/AFB signaling for auxin canalization and also show its importance for the regeneration of vascular strands and leaf venation.
Materials and Methods
Plant material and plant growth conditions
Wild‐type Col‐0 (NASC, The Nottingham Arabidopsis Stock Centre; http://www.arabidopsis.info/BasicForm) and reporter lines DR5rev::GFP (Friml et al., 2003) and pPIN1::PIN1:GFP (Benkova et al., 2003) produced in the Col‐0 background were used as controls. pin1‐1, tir1‐1, tir1‐1 afb2 afb3, arf7‐394 arf19‐1 and HS::axr3‐1 have been previously described (Knox et al., 2003; Sauer et al., 2006; Lavy & Estelle, 2016; Fendrych et al, 2016). tir1‐1 afb1 afb3 was produced by us for this study. All mutants and transgenic lines used in this study are in the Arabidopsis thaliana ecotype Columbia (Col‐0) background. Plants were germinated in pots with soil and vermiculite mixture (1 : 1, v : v). Seedlings with two pairs of true leaves were individually planted and grown in pots with soaked peaty rings in a growth chamber under long‐day light conditions at 20°C. Plants with inflorescence stems 10 cm tall were chosen for the experiments.
Local auxin application and vasculature regeneration experiments in Arabidopsis stems
Young plants with inflorescence stems having primary tissue architecture (vascular bundles separated by interfascicular parenchyma sectors) were chosen for the following two‐step experiments, according to the protocol of Mazur et al. (2016). First, the flowering parts of the stems were removed by using a sharp razor blade. The resulting stems (7 cm tall after dissection) were attached to a polypropylene tube to stiffen them and placed under a lead ball (2.5 g). The weight was applied for 6 d to produce a closed ring of cambium on the stem circumference (Mazur et al., 2014). Next, the samples were incised transversally above the leaf rosette, and a droplet of lanoline paste with auxin (IAA; Sigma‐Aldrich, cat. no. 15148‐2G) or auxin plus inhibitors (NPA (N‐1‐naphtylphthalamic acid), Sigma; PEO‐IAA (α‐(phenyl ethyl‐2‐one)‐indole‐3‐acetic‐acid; auxinole, Sigma)) was locally applied below the cut. The incision was made in the transverse plane to disturb the longitudinal continuum of cambium and polar, basipetal transport of endogenous auxin. We were thus certain that the analyzed changes are the results of the externally applied auxin only. The applied compounds were replaced during the experiments every 2 d with a fresh droplet. For local application, 10 µM water solutions of all compounds mixed with a droplet of lanolin paste were used. Stock solutions of auxin and inhibitors (NPA, auxinole, PEO‐IAA) were dissolved in dimethyl sulfoxide (cat. no. D5879‐500ML; Sigma). Experiments were conducted twice for each line, with at least 10 plants analyzed in each run. Finally, the samples were collected, manually sectioned and mounted in a 50% glycerol aqueous solution onto imaging glass.
Leaf and cotyledon clearing
To reveal their vasculature, leaves/cotyledons of 8‐d‐old plants were treated with the following: 70% ethanol (overnight at 4°C); 4% HCl + 20% methanol (12 min at 65°C); 7% NaOH + 60% ethanol (15 min at room temperature); HCl (10 min at room temperature); seedlings were rehydrated by successive incubations in 60/40/20/10% in ethanol for 10 min; and 5% ethanol + 25% glycerol (a few days at 4°C until the air bubbles within the tissue had disappeared).
Verification of transgenic line identity
The mutations in the genomes of the mutant plant lines used in this work were verified by PCR. Namely, genomic DNA was extracted from mechanically ground leaves of 3‐wk‐old plants and used as a template in PCR with wild type‐ and mutation‐specific primers. The presence of the tir1‐1 point mutation was tested via tir1 amplicon digestion with MboI endonuclease. Since regular PCR results were inconclusive for afb1 and afb3 insertional mutations in the tir1afb1afb3 mutant, reverse transcription PCR was used instead, with cDNA from total leaf RNA preparation used as a template.
Imaging and image analysis
Samples of wounded stems were analyzed via a stereomicroscope (Nikon MSZ1500) equipped with a charge‐coupled device (CCD) camera DS‐Fi1. The green fluorescent protein (GFP) reporter lines were analyzed using Zeiss Observer.Z1 and Olympus Fluoview FV1000 confocal laser‐scanning microscopes. GFP fluorescence was excited by an argon‐ion laser light of 488 nm, detected at 510 nm. Acquired images were processed with ZEN 2012 Light Edition and fluoview software. Transmitted light observations were made via an Olympus BX43 microscope equipped with Olympus SC30 camera. Figures were created with coreldraw X6.
Quantification and statistical analysis
All calculations and graphs were made with Microsoft Office excel software. Unpaired Student's t‐tests (P < 0.05) and one‐way ANOVA were used to compare sets of data (P < 0.0001). Error bars in the graphs indicate standard errors.
Results
Vasculature regeneration after wounding requires TIR1/AFB signaling
Previously (Mazur et al., 2016), we showed that stem vasculature regeneration after wounding was associated with the activation of nuclear auxin signaling and induction of PIN1 auxin transport channels. In the present work, we wanted to test if this regeneration was dependent on the latter two factors.
To this end, we wounded inflorescence stems of Col‐0 as well as triple tir1afb1afb3 and tir1afb2afb3 mutants and assessed the extent of vasculature regeneration in them 6 d after wounding (DAW; Fig. 1a,b). Double ARF (arf7arf19) mutants were analyzed as well, because these particular ARFs have been shown previously to be required for auxin signaling and auxin‐mediated re‐arrangements of polar PIN1 distribution in roots (Okushima et al., 2005; Sauer et al., 2006). While two modes of vascular strand formation, namely passing around the wound and through the callus forming within the wound (both composed of elongated cells with stripes of secondary cell wall features) were present in the majority of sectioned Col‐0 stems, only the vasculature passing through callus was visible in all tir/afb triple mutant samples (Fig. 1c–f). All arf7arf19 mutant samples lacked callus formation after injury and did not regenerate vasculature around the wound (Fig. 1g).
Figure 1.
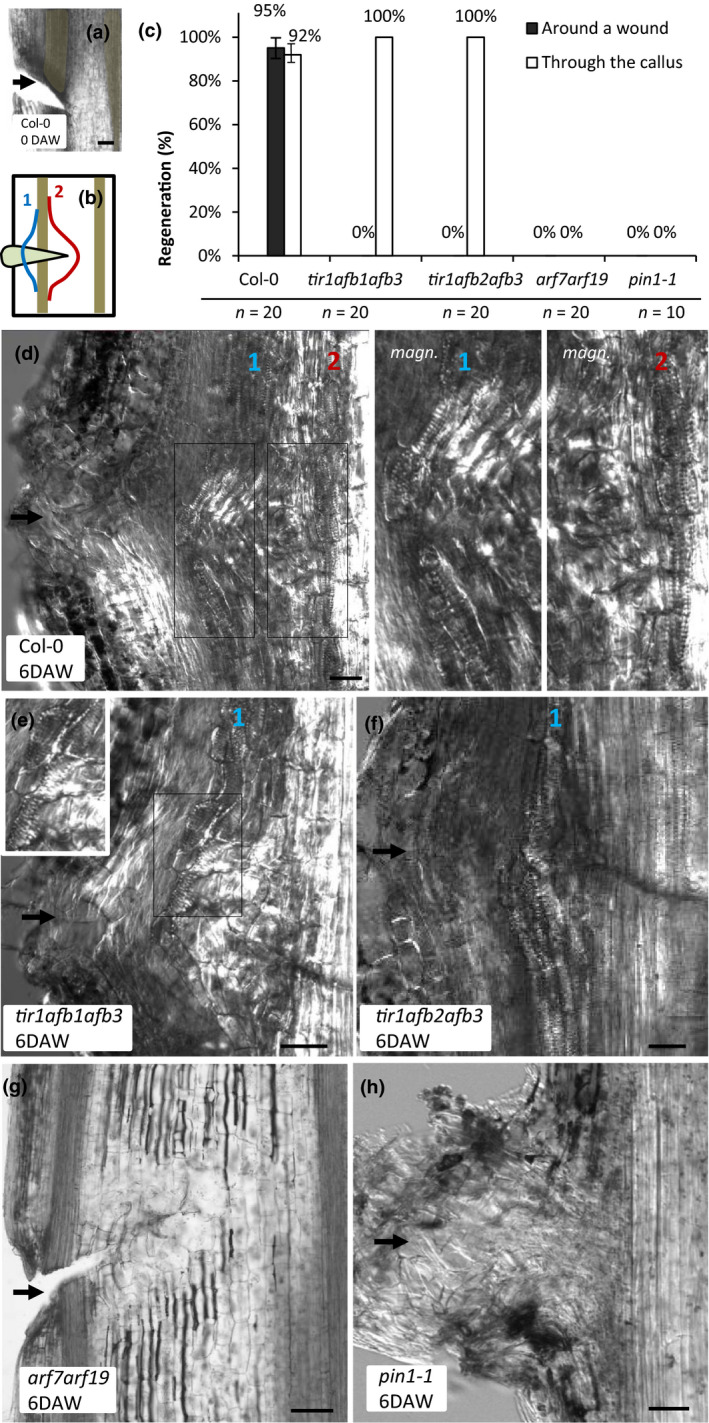
Vasculature regeneration after wounding in tir1/afb, arf7arf19 and pin1‐1 mutants of Arabidopsis. (a) Longitudinal section through a Col‐0 inflorescence stem 8 h after wounding before callus formation. Black arrow indicates the approximate location of the wound. (b) Schematic depiction of vasculature regeneration after wounding in Arabidopsis inflorescence stem. Two brown vascular strands are shown similar to (a). The left one is broken due to wounding. The wound is filled with callus (light brown). (c) Frequencies of vasculature regeneration after wounding in stems of different genotypes. Error bars indicate SE. (d–h) Examples of wounded Col‐0, tir1afb1afb3, tir1afb2afb3, arf7arf19 and pin1‐1 inflorescence stems 6 d after wounding (DAW). Black arrows show the approximate locations of wounding sites. White rectangles specify which parts of the images are magnified to make the secondary cell wall features of regenerated vascular cells more apparent. Blue (‘1’) and red (‘2’) numbers mark the regenerated vascular strands passing through callus and around the wound, respectively. Bars, 50 µm (a, d–h). See also Supporting Information Fig. S1.
In addition to these constitutive mutants, we analyzed HS::axr3‐1 plants, in which the expression of a dominant negative form of the IAA17/AXR3 transcriptional repressor can be induced by thermal stress (Knox et al., 2003; Hayashi, 2012). After the stems were wounded, HS::axr3‐1 induction was conducted by incubating the plants at 37°C for 1 h every day, which strongly inhibited vasculature regeneration and callus formation. In particular, no vasculature formed around the wound in 70% (14/20) of samples 6 DAW. In the remaining 30% of stems (6/20), groups of cells with denser cell walls were present above the wound 6 DAW (Supporting Information Fig. S1). They were never elongated or arranged into well‐defined strands and lacked the signs of secondary cell wall patterning.
To study the importance of PIN1, the same experiments were conducted on pin1‐1 knockout plants. The results were similar, with no vasculature passing through callus or around the wound visible 6 DAW in all samples (Fig. 1h).
Thus, our analysis of vasculature regeneration after wounding in constitutive and inducible mutants revealed that while PIN1‐mediated auxin transport and TIR1/AFB auxin perception are required for vasculature regeneration around the wound, vasculature can still regenerate through callus when either one of these two processes is suppressed.
Local auxin application induces TIR1/AFB‐ and PIN1‐dependent vascular strand development in Arabidopsis
Having observed a failure of tir1/afb mutants to regenerate vasculature after wounding, we wanted to test if this was due to the direct involvement of TIR1/AFB auxin perception in auxin canalization rather than in other regeneration‐associated processes. For this, we complemented our wounding protocol with local auxin application. This is a classical experimental setup, which allows us to induce formation of auxin channels specifically by auxin treatment. In particular, although wounding is required in these experiments to stop the normal flow of auxin, its canalization is not induced unless the injury is accompanied by local auxin application below the cut site (Fig. 2a,b).
Figure 2.
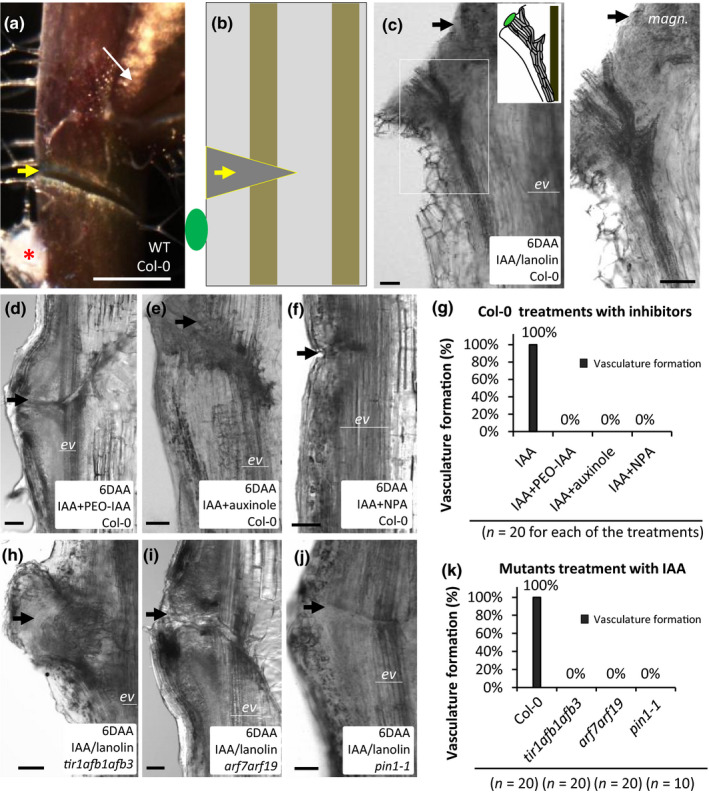
Vascular strands formation in response to local auxin application onto Arabidopsis stems. (a) Fragment of a wounded Col‐0 inflorescence stem. Auxin (with or without various inhibitors) was locally applied in a droplet of lanolin paste below the wound (red asterisk). Axillary buds above the wound were not removed in the experiments (narrow white arrow). (b) Schematic representation of (a) on a longitudinal section. Compounds are applied in a droplet of lanolin paste below the wound (green oval). The droplet is removed from the sample before sectioning for technical reasons and therefore cannot be located on the actual images. The vascular strand is broken by a transverse cut. (c–f) Examples of longitudinal sections of Col‐0 stems 6 d after local application (DAA) of IAA alone or its co‐application with PEO‐IAA, auxinole or NPA. (h–j) Examples of longitudinal sections of tir1afb1afb3, arf7arf19 and pin1‐1 stems 6 d after local application of IAA. (g, k) Frequencies of vascular strands formation under the studied experimental conditions. Thick arrows (yellow or black) indicate the approximate location of wounds. The bottom fragments of broken vascular strands (pre‐existing vasculature) are labeled ‘ev’. Bars: (a) 1mm; (c–f, h–j) 50 µm.
Application of natural auxin (IAA) dissolved in lanolin wax (100 nM) onto the surface of wounded Col‐0 inflorescence stems led to the formation of thick vasculature (appearing as black strands extending downwards from the periphery into the deeper regions of the tissue) 6 d after auxin application (DAA) in almost 80% (32/40) of samples (Fig. 2c).
To test if this phenomenon depended on TIR1/AFB signaling and PIN1‐mediated auxin transport, we treated wounded Col‐0 stems with local co‐application of auxin and inhibitors of either TIR1/AFB signaling (auxinole, PEO‐IAA) or auxin transport (NPA) in the same drop of wax (Hayashi et al., 2012). No vascular strands developed around the auxin application site 6 DAA under such experimental conditions (Fig. 2d–g). Local application of auxin onto the stems of tir1afb1afb3, arf7arf19 and pin1‐1 mutants yielded similar results (Fig. 2h–k).
Thus, via a combination of genetic and pharmacological approaches, we show that TIR1/AFB signaling and PIN1‐dependent auxin transport are required for vascular strands development in response to local auxin application in Arabidopsis inflorescence stems.
Local auxin application induces TIR1/AFB‐ and PIN1‐dependent auxin canalization in Arabidopsis
To validate that vascular strand formation in response to local auxin application represents auxin canalization, we visualized auxin response and polar auxin transport in the established experimental system via the genetic markers DR5rev::GFP (Friml et al., 2003) and pPIN1::PIN1‐GFP (Benkova et al., 2003), respectively.
Local auxin application resulted in DR5 activation at the application site 8 h after auxin application (HAA; Fig. 3a). GFP‐positive cells were arranged in a strand, similar to the vasculature in Fig. 2(c). At 4 DAA, a wide field of bright GFP fluorescence (Fig. S2a) and a narrow channel of cells expressing PIN1‐GFP (Fig. 3b) were observed between the organ periphery and the pre‐existing stem vasculature. Much weaker induction of DR5rev::GFP and PIN1‐GFP expression was visible 4 and 6 DAA near the application site, when the stems were locally co‐treated with IAA and TIR1/AFB inhibitors or NPA (Fig. 3c–i; Fig. S2b,c). In particular, green cells did not form defined strands under these experimental conditions and instead were found at the periphery of the organ.
Figure 3.
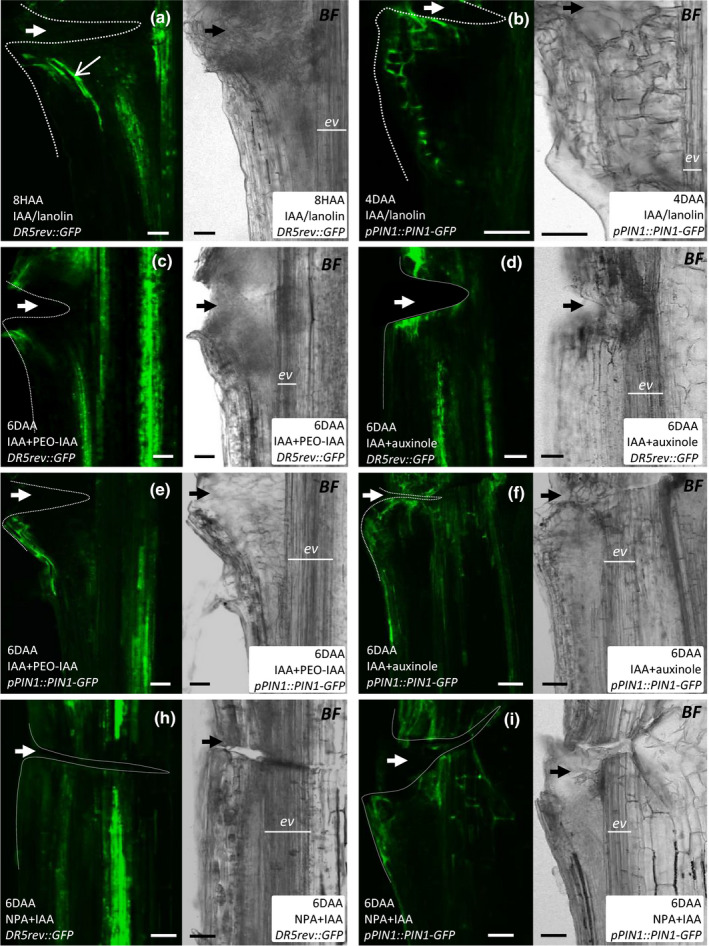
Requirement of TIR1/AFB auxin perception and PIN‐mediated auxin transport in auxin canalization induced by local auxin application onto Arabidopsis stems. (a–i) Examples of longitudinal sections through DR5rev::GFP and pPIN1::PIN1‐GFP stems obtained after local application of IAA alone or along with PEO‐IAA, auxinole or NPA. Each section was imaged in green and bright field channels. Dotted lines indicate the wounded stem regions. Thick arrows (white or black) indicate the approximate location of wounds. Bars, 50 µm. See also Supporting Information Fig. S2.
Thus, these data show that local auxin application onto Arabidopsis inflorescence stems induces formation of PIN1‐positive, high‐auxin response channels from the exogenous source towards the pre‐existing stem vasculature, which is blocked by pharmacological inhibition of either auxin perception or its directional transport.
TIR1/AFB signaling is required for proper leaf venation and auxin‐induced PIN1 lateralization in the root
To complement our wounding and local auxin application observations with less invasive experiments, we analyzed other, spontaneously occurring auxin canalization‐related physiological processes.
First, we looked at leaf venation, because it requires PIN‐dependent auxin transport and is accompanied by the formation of DR5/PIN1‐positive channels (Scarpella et al., 2006; Sawchuk & Scarpella, 2013), similar to the case of auxin canalization from the exogenous source. We observed that two triple mutants defective in TIR1/AFB‐mediated auxin perception (tir1afb1afb3 and tir1afb2afb3) exhibited strong leaf venation defects in cotyledons (Fig. 4a) and primary leaves (Fig. S4). The strong abnormalities included apical disconnections between the central and lateral veins in cotyledons and the lack of one or both lateral veins in leaves (Fig. 4b). Thus, the analysis of leaf/cotyledon vasculature in tir1/afb mutants evidently shows that although vasculature can form, its intricate, organized pattern during leaf venation strongly depends on TIR1/AFB auxin perception.
Figure 4.
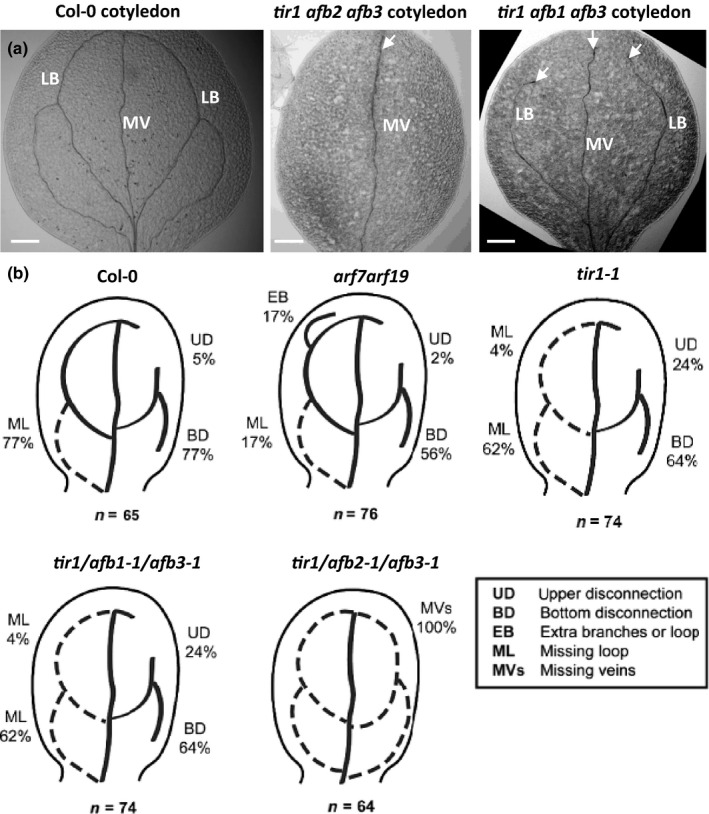
Cotyledon venation in TIR1/AFB mutants of Arabidopsis. (a) Vasculature defects in Col‐0, tir1afb2afb3 and tir1afb1afb3 cotyledons. Arrowheads highlight vasculature defects of the lateral branches (LB) and the middle vein (MV). (b) Schemes demonstrating the types and frequencies of venation abnormalities observed. Bars, 200 µm (a). See also Supporting Information Figs S3 and S4.
To obtain some glimpses into which cellular processes during canalization are targeted by TIR1/AFB signaling, we analyzed PIN1 re‐localization in the root tip cells occurring in response to a relatively short (4 h) auxin treatment (Sauer et al., 2006; Prat et al., 2018). This phenomenon has an unclear physiological significance but provides a simple assessment of auxin's effect on PIN polarity – one of the key prerequisites of canalization. Notably, it also asseses this effect without any obvious accompanying morphological changes or cell fate re‐specification processes occurring during vasculature formation. Normally in this case, the intracellular localization of PIN1 shifts from a predominantly basal position to the inner lateral side of endodermal and pericycle cells. However, we found the roots of tir1afb2afb3 plants to be much less responsive to auxin in terms of this PIN1 lateralization (Fig. S3a,b), which is consistent with similar, previously published observations in axr3 and arf7arf19 mutants (Sauer et al., 2006).
In summary, these observations demonstrate that TIR1/AFB signaling is important for auxin canalization not only under more invasive experimental conditions, such as wounding and local auxin application, but also in an undisturbed, physiological process involving vasculature formation such as leaf venation. The TIR1/AFB signaling may act on auxin‐mediated PIN1 repolarization, as suggested by defects in this process in the roots of mutants defective in this auxin signaling pathway.
Discussion
The vascular tissue network crucially aids plants to thrive in almost all land habitats. The mechanism of its formation is intriguing not only due to the importance of vasculature for plant life altogether, but also due to its reiterative nature and developmental flexibility (it can be induced in many contexts, such as during generation of nascent organs or regeneration after wounding). These properties of vasculature formation are explained by a self‐organizing nature of auxin canalization – an organized establishment of auxin transport channels from localized auxin sources. The most evident manifestation of auxin canalization can be observed in classical experiments involving the induction of a canalized auxin flow away from the site of its local application (Sachs, 1975, 1981; Berleth & Sachs, 2001; Sauer et al., 2006; Balla et al., 2011; Sawchuk & Scarpella, 2013; Bennett et al., 2014; Adamowski & Friml, 2015; Cieslak et al., 2015; Mazur et al., 2016). This process is known to involve intracellular polarization of PIN auxin transporters, which is coordinated between individual vasculature progenitors in a way that ultimately generates auxin‐transporting channels. Details of the molecular and cell biological mechanisms of this coordinated polarization are missing, however. The classical canalization hypothesis proposes the existence of a positive feedback interaction between auxin perception and the regulation of its intercellular transport direction as determined by the cellular PIN polarities (Vieten et al., 2005; Adamowski & Friml, 2015).
In the past, verification of this idea was difficult because of the limitations of transgenesis in the species where local auxin application experiments were possible. In the present study, we demonstrate that the classical methodology can be successfully applied to the classical genetic model A. thaliana with the available large collection of mutants and marker lines. The local auxin application experimental setup has several significant advantages over that previously used to study canalization, such as vasculature regeneration after wounding, as it allows us to: exclude the potential confounding factors associated with stem wounding (because the induction of auxin canalization is achieved by auxin application per se); control the dosage of the inductive stimulus (auxin concentration and duration of its supplementation); and test the inductive potential of various auxin analogs and/or complement the induction of auxin canalization with the effects of other bioactive compounds (e.g. inhibitors).
In addition, we show that auxin canalization under the improved experimental setup depends strictly on PIN1‐mediated transport and TIR1/AFB signaling, providing a necessary demonstration of their presumed involvement. The fact that TIR1/AFB signaling is also required for proper progression of auxin canalization‐related processes under more physiological conditions, such as vasculature regeneration after wounding, leaf venation and PIN1 lateralization in the root, suggests that the results derived from the proposed methodology are likely to be not idiosyncratic but of general relevance.
That being said, it would be interesting to test if other cases of vasculature development, such as those occurring during graft transplantation (Melnyk et al., 2015) and organ regeneration from cell culture (Kareem et al., 2015), share this requirement. Furthermore, our experimental system may be used to characterize the role of those auxin signaling components, which have been reported to be important for leaf venation (Donner et al., 2009), under simpler and more controllable auxin canalization conditions.
At the same time, the suggested methodology has certain limitations. In particular, it provides no dynamic, live information, as the samples, due to their thickness and opacity, need to be mechanically sectioned to allow microscopy. For the same reason, it is not trivial to characterize the complete 3D distribution of vasculature and fluorescent reporters, although a more refined method of sectioning compared to that used in this research should alleviate this restriction.
With the present work, we hope to re‐ignite the classical studies of auxin canalization using modern transgenesis and imaging techniques. In particular, hypotheses on the interaction between auxin perception and its polar transport could be tested via local auxin application in particular mutants. For example, various cellular processes such as endocytosis protein recycling or degradation may be genetically and pharmacologically manipulated to probe their role in auxin canalization (Wabnik et al., 2010; Grones et al., 2015). Although not perfect, we think that the suggested methodology would help towards the understanding of how individual plant cells communicate with one another to achieve coordinated tissue polarization and how the auxin‐transporting channels activate the downstream developmental programs of vasculature differentiation.
Author contributions
EM, IK, JH and JF designed and conducted experiments and analyzed the data. EM and JF wrote the manuscript, with the assistance of IK.
Supporting information
Fig. S1 Defects in vasculature regeneration in wounded HS::axr3‐1 Arabidopsis mutant.
Fig. S2 DR5rev::GFP and pPIN::PIN1‐GFP fluorescence distribution at additional time points after local compound application.
Fig. S3 PIN1 lateralization in the roots of Col‐0, HS::axr3‐1, arf7arf19 and tir1afb2afb3 genotypes in response to NAA treatment.
Fig. S4 Abnormal venation in primary leaf of tir1afb2afb3 Arabidopsis mutant.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank Mark Estelle, José M. Alonso and the Arabidopsis Stock Centre for providing seeds. We acknowledge the core facility CELLIM of CEITEC supported by the MEYS CR (LM2015062 Czech‐BioImaging) and Plant Sciences Core Facility of CEITEC Masaryk University for help in generating essential data. This project received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 742985) and the Czech Science Foundation GAČR (GA13‐40637S and GA18‐26981S) to JF. JH is the recipient of a DOC Fellowship of the Austrian Academy of Sciences at the Institute of Science and Technology. The authors declare no competing interests.
Contributor Information
Ewa Mazur, Email: ewa.mazur@us.edu.pl.
Ivan Kulik, Email: ivan.kulik@sund.ku.dk.
References
- Adamowski M, Friml J. 2015. PIN‐dependent auxin transport: action, regulation and evolution. The Plant Cell 27: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla J, Kalousek P, Reinohl V, Friml J, Prochazka S. 2011. Competitive canalization of PIN‐dependent auxin flow from axillary buds controls pea bud outgrowth. The Plant Journal 65: 571–577. [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G, Friml J. 2003. Local, efflux‐dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- Bennett T, Hines G, Leyser O. 2014. Canalization: what the flux? Trends in Genetics 30: 41–48. [DOI] [PubMed] [Google Scholar]
- Berleth T, Mattsson J, Hardtke CS. 2000. Vascular continuity and auxin signals. Trends in Plant Science 5: 387–393. [DOI] [PubMed] [Google Scholar]
- Berleth T, Sachs T. 2001. Plant morphogenesis: long‐distance coordination and local patterning. Current Opinion in Plant Biology 4: 57–62. [DOI] [PubMed] [Google Scholar]
- Bhatia N, Bozorg B, Larsson A, Ohno C, Jönsson H, Heisler MG. 2016. Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis. Current Biology 26: 3202–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano‐Delgado A, Lee JY, Demura T. 2010. Regulatory mechanisms for specification and patterning of plant vascular tissues. Annual Review of Cell Developmental Biology 26: 605–637. [DOI] [PubMed] [Google Scholar]
- Cieslak M, Runions A, Prusinkiewicz P. 2015. Auxin‐driven patterning with unidirectional fluxes. Journal of Experimental Botany 66: 5083–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Elo A, Helariutta Y. 2009. Hormone interactions during vascular development. Plant Molecular Biology 69: 347–360. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Estelle M. 2004. Auxin signaling and regulated protein degradation. Trends in Plant Science 9: 302–308. [DOI] [PubMed] [Google Scholar]
- Donner TJ, Sherr I, Scarpella E. 2009. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 136: 3235–3246. [DOI] [PubMed] [Google Scholar]
- Fendrych M, Leung J, Friml J. 2016. TIR1/AFB‐Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5: e19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. 2003. Efflux‐dependent auxin gradients establish the apical–basal axis of Arabidopsis . Nature 426: 147–153. [DOI] [PubMed] [Google Scholar]
- Grones P, Chen X, Simon S, Kaufmann WA, De Rycke R, Nodzynski T, Zazimalova E, Friml J. 2015. Auxin‐binding pocket of ABP1 is crucial for its gain‐of‐function cellular and developmental roles. Journal of Experimental Botany 66: 5055–5065. [DOI] [PubMed] [Google Scholar]
- Hayashi KI. 2012. The interaction and integration of auxin signaling components. Plant Cell Physiology 53: 965–975. [DOI] [PubMed] [Google Scholar]
- Hayashi KI, Neve J, Hirose M, Kuboki A, Shimada Y, Kepinski S, Nozaki H. 2012. Rational design of an auxin antagonist of the SCFTIR1 auxin receptor complex. ACS Chemical Biology 7: 590–598. [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current Biology 15: 1899–1911. [DOI] [PubMed] [Google Scholar]
- Kareem A, Durgaprasad K, Sugimoto K, Du Y, Pulianmackal AJ, Trivedi ZB, Abhayadev PV, Pinon V, Meyerowitz EM, Scheres B et al 2015. PLETHORA genes control regeneration by a two‐step mechanism. Current Biology 25: 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K, Grierson CS, Leyser O. 2003. AXR3 and SHY2 interact to regulate root hair development. Development 130: 5769–5777. [DOI] [PubMed] [Google Scholar]
- Lavy M, Estelle M. 2016. Mechanisms of auxin signaling. Development 143: 3226–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M. 2016. Constitutive auxin response in Physcomistrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur E, Benkova E, Friml J. 2016. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Scientific Reports 6: e33754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur E, Kurczyńska EU, Friml J. 2014. Cellular events during interfascicular cambium ontogenesis in inflorescence stems of Arabidopsis . Protoplasma 251: 1125–1139. [DOI] [PubMed] [Google Scholar]
- Melnyk CW, Schuster C, Leyser O, Meyerowitz EM. 2015. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana . Current Biology 25: 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D et al 2005. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19 . The Plant Cell 17: 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat T, Hajny J, Grunewald W, Vasileva M, Molnar G, Tejos R, Schmid M, Sauer M, Friml J. 2018. WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity. PLoS Genetics 14: e1007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusova H, Abbas M, Han H, Song S, Robert HS, Friml J. 2016. Termination of shoot gravitropic responses by auxin feedback on PIN3 polarity. Current Biology 26: 3026–3032. [DOI] [PubMed] [Google Scholar]
- Raven JA. 1975. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients and its significance for polar IAA transport. New Phytologist 74: 163–172. [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso J, Weijers D, Friml J. 2013. Local auxin sources orient the apical–basal axis in Arabidopsis embryos. Current Biology 23: 2506–2512. [DOI] [PubMed] [Google Scholar]
- Sachs T. 1975. The induction of transport channels by auxin. Planta 127: 201–206. [DOI] [PubMed] [Google Scholar]
- Sachs T. 1981. The control of the patterned differentiation of vascular tissues. Advances in Botanical Research 9: 151–262. [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wiśniewska J, Reinöhl V, Friml J, Benkova E. 2006. Canalization of auxin flow by Aux/IAA‐ARF‐dependent feedback regulation of PIN polarity. Genes Development 20: 2902–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk MG, Scarpella E. 2013. Polarity, continuity, and alignment in plant vascular strands. Journal of Integrative Plant Biology 55: 824–834. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. 2006. Control of leaf vascular patterning by polar auxin transport. Genes Development 20: 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Puech L, Regan S, Fink S, Olsson O, Sundberg B. 2000. Cambial‐region‐specific expression of the Agrobacterium iaa genes in transgenic aspen visualized by a linked uidA reporter gene. Plant Physiology 123: 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EJ, Sundberg B. 1998. Indole‐3‐acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiology 117: 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. 1996. Auxin as a positional signal in pattern formation in plants. Proceedings of the National Academy of Sciences, USA 93: 9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, Benkova E, Benjamins R, Beeckman T, Luschnig C, Friml J. 2005. Functional redundancy of PIN1 proteins is accompanied by auxin dependent cross‐regulation of PIN expression. Development 132: 4521–4531. [DOI] [PubMed] [Google Scholar]
- Wabnik K, Kleine‐Vehn J, Balla J, Sauer M, Naramoto S, Reinöhl V, Merks RM, Govaerts W, Friml J. 2010. Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Molecular Systems Biology 6: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CI, Schuetz M, Yu Q, Mattsson J. 2007. Dynamics of MONOPTEROS and PIN‐FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana . The Plant Journal 49: 387–398. [DOI] [PubMed] [Google Scholar]
- Zhang J, Nodzynski T, Pencik A, Rolcik J, Friml J. 2010. PIN phosphorylation is sufficient to mediate polarity and direct auxin transport. Proceedings of the National Academy of Sciences, USA 12: 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Defects in vasculature regeneration in wounded HS::axr3‐1 Arabidopsis mutant.
Fig. S2 DR5rev::GFP and pPIN::PIN1‐GFP fluorescence distribution at additional time points after local compound application.
Fig. S3 PIN1 lateralization in the roots of Col‐0, HS::axr3‐1, arf7arf19 and tir1afb2afb3 genotypes in response to NAA treatment.
Fig. S4 Abnormal venation in primary leaf of tir1afb2afb3 Arabidopsis mutant.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


