Abstract
Background
Anti‐platelet antibody testing may be useful for the diagnosis and management of childhood immune thrombocytopenia (ITP).
Objectives
Here we aimed to assess the prevalence and prognostic significance of anti‐platelet glycoprotein‐specific IgM and IgG antibodies.
Methods
Children with newly diagnosed ITP were included at diagnosis and randomized to an intravenous immunoglobulins (IVIg) or careful observation group (TIKI trial). In this well‐defined and longitudinally followed cohort (N = 179), anti‐platelet glycoprotein‐specific IgM and IgG antibodies were determined by monoclonal antibody‐immobilization of platelet antigens.
Results
The dominant circulating anti‐platelet antibody class in childhood ITP was IgM (62% of patients); but IgG antibodies were also found (10%). Children without IgM platelet antibodies were older and more often female. There was weak evidence for an association between IgM anti‐GP IIb/IIIa antibodies and an increased bleeding severity (P = .03). The IgM and IgG anti‐platelet responses partially overlapped, and reactivity was frequently directed against multiple glycoproteins. During 1‐year follow‐up, children with IgM antibodies in the observation group displayed a faster platelet recovery compared to children without, also after adjustment for age and preceding infections (P = 7.1 × 10−5). The small group of patients with detectable IgG anti‐platelet antibodies exhibited an almost complete response to IVIg treatment (N = 12; P = .02), suggesting that IVIg was particularly efficacious in these children.
Conclusions
Testing for circulating anti‐platelet antibodies may be helpful for the clinical prognostication and the guidance of treatment decisions in newly diagnosed childhood ITP. Our data suggest that the development of even more sensitive tests may further improve the clinical value of antibody testing.
Keywords: autoantibodies, immune thrombocytopenia, intravenous immunoglobulins, pediatrics, platelets
Essentials.
Glycoprotein‐specific IgM platelet antibodies are the dominant antibody class in childhood ITP.
IgM platelet antibodies associate with spontaneous recovery from ITP.
IgG platelet antibodies associate with a favorable response to IVIg.
Testing for platelet antibodies may be helpful to determine disease courses already at diagnosis.
1. INTRODUCTION
Childhood immune thrombocytopenia (ITP) is a rare autoimmune bleeding disorder with an incidence of 2 to 6 children per 100 000 annually,1 characterized by a platelet count below 100 × 109/L.2 About 50% of children experience an infectious episode before the onset of bleeding symptoms and thrombocytopenia, and it is hypothesized that epitope spreading or molecular mimicry may play a role in the development of self‐directed anti‐platelet immunity.3, 4, 5 The pathophysiology of ITP features both humoral and cellular immune responses against platelet self‐antigens.6 Thus, anti‐platelet antibody testing could potentially be used in the diagnosis and determination of prognosis of ITP.
A pathophysiological role of anti‐platelet antibodies in ITP has been demonstrated by the identification of a transferable factor in the plasma of adult ITP patients, which induced thrombocytopenia in healthy individuals. This factor was located in the 7S fraction of human gamma globulin and could be adsorbed to platelets.7, 8 Studies using platelets from patients with Glanzmann disease have identified the first platelet antigen targeted by ITP anti‐platelet antibodies as the fibrinogen receptor glycoprotein (GP) IIb/IIIa.9 It is now known that multiple platelet glycoproteins can be involved, including GP V and the GP Ib/IX complex.10, 11 In childhood ITP, IgM anti‐platelet antibodies have been suggested to be at least as prevalent as anti‐platelet IgG antibodies.12, 13, 14, 15 Apart from affecting platelet clearance, anti‐glycoprotein antibodies can functionally impair platelet aggregation by blocking the fibrinogen receptor interactions,16, 17 or may potentially inhibit effective thrombopoiesis through GPIbα‐mediated hepatocyte thrombopoietin production.18
While early antibody tests have focused on the detection of antibodies bound to whole platelets,19, 20 this approach has been dismissed because of the unspecific adsorption of protein to platelets, and false‐positive results in non‐immune mediated thrombocytopenic patients.21, 22, 23 Improved antigen‐specific antibody tests were subsequently developed, for example by monoclonal antibody‐immobilization of platelet antigens (MAIPA)24 or immunobead technology,25 and these have today largely replaced whole‐platelet tests with improved specificity.23, 26 The first glycoprotein‐specific study amongst 15 children with acute ITP identified IgG anti‐GP IIb/IIIa in 27% of patients, and detected no anti‐GP Ib/IX antibodies.25 More recently, IgG glycoprotein‐specific antibodies were investigated in 74 children with newly diagnosed and treatment‐naive ITP.27 IgG anti‐GP IIb/IIIa and anti‐GP Ib/IX antibodies were detected among 36% and 30% of children, respectively. This study found no association with transient versus chronic disease courses. Unfortunately, inclusion criteria, controls, clinical characteristics, and follow‐up of included patients were not described, making it difficult to put these data in clinical perspective. In contrast, a Chinese study suggested that anti‐GP Ib/IX antibodies in children with newly diagnosed ITP might be associated with the development of chronic ITP, whereas anti‐GP IIb/IIIa antibodies may be associated with transient disease.28 Although these data are suggestive of a potential use of anti‐platelet antibody testing, the diagnostic and prognostic value of such testing remains unclear.29, 30
In the present study, we sought to provide definitive evidence by evaluating the prevalence and prognostic significance of glycoprotein‐specific anti‐platelet antibody testing performed in our national reference laboratory in a clinically well‐described, large cohort of children with newly diagnosed ITP, who were followed longitudinally for 1 year.
2. METHODS
2.1. Study participants
We report data from the phase 3 randomized controlled, open‐label trial Therapy with or without Intravenous Immunoglobulins for Newly Diagnosed Immune Thrombocytopenia in Kids in the Netherlands (TIKI).31 The study protocol was approved by the ethical review board of University Medical Center Utrecht and all participating sites, and conducted in accordance with the second Declaration of Helsinki and Good Clinical Practice guidelines. Parents or patients aged ≥12 years gave written informed consent for study participation. Children with newly diagnosed ITP (N = 200) were allocated in a 1:1 ratio to be administered 0.8 g/kg intravenous immunoglobulin or followed by careful observation. The randomization into the trial arms was stratified by a platelet count below or above 10 × 109/L. To be eligible for inclusion, patients had to be 3 months to 16 years of age, with newly diagnosed ITP in the past 72 hours, according to guidelines,2, 30 a platelet count ≤20 × 109/L and bleeding symptoms (Buchanan score 1‐3). Exclusion criteria were severe bleeding with an indication for IVIg therapy (Buchanan score 4‐5), immune modulatory treatment within the past month, or insufficient command of language. The primary outcome of the trial was development of chronic ITP. Secondary outcomes included bleeding symptoms, resolution of thrombocytopenia, and biological factors related to the pathophysiology of ITP and response to IVIg. Blood samples were collected at study sites and transferred to a central laboratory facility (Laboratory for Platelet and Leukocyte Serology; Sanquin Diagnostics).
Samples from children with chronic ITP were obtained from the multicenter cross‐sectional CINKID study (Chronic ITP in the Netherlands in Kids).32 Inclusion criteria of the study were an age between 6 months to 17 years. Children were excluded for presence of autoimmune phenomena, other cytopenia, or features suggestive of hereditary thrombocytopenia. Parents or patients aged ≥12 years gave written informed consent for study participation. The study was approved by the ethical review board of University Medical Center Utrecht.
Control samples were obtained from healthy volunteers (Sanquin), adult refractory ITP patients included in the HOVON64 study,33 juvenile idiopathic arthritis (JIA) patients who were in remission, and patients with autoimmune neutropenia sent for anti‐neutrophil antibody evaluation (Sanquin). The study was conducted in agreement with Dutch national guidelines (Human Tissue and Medical Research: Code of conduct for responsible use; https://www.federa.org/codes‐conduct) and the Declaration of Helsinki. All analyses were performed on coded, de‐identified data. Serum and plasma samples were stored at −20°C.
2.2. Clinical outcome definition
Bleeding symptoms were recorded on a modified Buchanan score.34 The recovery from thrombocytopenia was defined according to recommendations by the International Working Group on ITP.2 A complete response was defined as a platelet count ≥100 × 109/L.
2.3. Glycoprotein‐specific IgM and IgG anti‐platelet antibody profiling
The platelet antigen specificity and semi‐quantitative levels of anti‐platelet antibodies of the IgM and IgG classes were determined using the monoclonal antibody immobilization of platelet antigens (MAIPA) assay,24 as follows. Goat anti‐mouse IgG‐Fc (Jackson ImmunoResearch) was coated overnight in 96‐well microplates (Nunc Maxisorb) at 3 µg/mL in 50 µL coating buffer at 4°C and blocked afterward with 0.2% BSA in 0.9% NaCl (w/v). Cryopreserved healthy human donor platelets from three individuals typed for human platelet antigens were washed and pelleted in U‐bottom 96‐well plates (Greiner). Washing of platelets was performed at 550 g for 5 minutes. The cells were then suspended in 50 µL PBS/2% BSA and incubated with 120 µL test serum or plasma for 30 minutes at 37°C. Subsequently, the plate was washed three times and incubated with 50 µL mouse anti‐human antibody against glycoprotein IIb/IIIa (CD61; CLB‐Thromb/1, C17, Sanquin; Y2.51 distributed in the Fourth International Workshop and Conference on Human Leukocyte Differentiation Antigens; Dr Cordell, John Radcliffe Hospital, Oxford, UK), glycoprotein Ibα (CD42b; CLB‐MB45, Sanquin) or glycoprotein V (CD42d; CLB‐SW16, Sanquin) for 30 minutes at 37°C. After three washing steps the cells were lysed with NP40 in 100 µL TRIS solubilization buffer in a fresh V‐bottom 96‐well plate (Nunc) for 30 minutes at 4°C. The plate was then centrifuged at 1400 g for 15 minutes. Supernatant (80 µL) was transferred into fresh reagent tubes, and mixed with 110 µL TRIS washing buffer containing 2% bovine serum albumin (BSA). The goat‐anti‐mouse IgG coated plate was washed five times with phosphate buffered solution (PBS)/Tween 0.05%. After this, 50 µL of the diluted supernatant was added to each well and the plate was incubated overnight at 4°C. The plate was then washed again and 50 µL goat anti‐human IgG (Fcγ)‐HRP or anti‐human IgM (Fcµ)‐HRP (Jackson ImmunoResearch) was added and incubated for 2 hours at 4°C. After five washing steps, the color reaction was started by addition of 50 µL substrate solution (o‐phenylenediamine dihydrochloride [DAKO] supplemented with H2O2 in H2O) and subsequently stopped by addition of 50 µL 4 N H2SO4. The optical density was measured on an Anthos‐HTII plate reader at 492 nm. All samples were tested in duplicate. The concentrations of mouse anti‐human, goat anti‐mouse, goat‐anti human‐HRP antibodies were determined against existing stocks and varied per batch. The monoclonal antibodies C17, Y2, MB45 and SW16 were produced and affinity‐purified from hybridomas (Department of Experimental Immunohematology; Sanquin Research) and validated against commercially available antibodies (Sanquin Reagents).
2.4. Statistical analyses
Data was analyzed in R 3.6.0 (R Core Team).35 Continuous variables were tested with a non‐parametric Wilcoxon rank test or Welch's t test, as appropriate. Multiple groups were compared by Kruskal‐Wallis test with a post‐hoc Nemenyi test. Frequencies were compared with a Chi‐squared test or Fisher’s exact test. Repeated measurements of platelet counts were analyzed using linear mixed effects models with random effects for the patient and fixed effects for platelet antibody status (positive/negative) and any covariates, as reported. A two‐sided P‐value below .05 was considered significant.
3. RESULTS
3.1. IgM anti‐platelet antibodies are directed against multiple glycoproteins in childhood ITP
We evaluated the presence of glycoprotein‐specific anti‐platelet antibodies in 179 children with newly diagnosed ITP. The median age was 4 years and 53% had experienced a preceding infection (Table 1); 96 children were randomized to treatment with IVIg and 86 to receive careful observation only. Due to the limited sample volume obtained from juvenile patients and the low platelet counts in ITP, we evaluated the presence of circulating anti‐platelet antibodies (indirect test), but not of anti‐platelet antibodies directly bound to patients' cells (direct test).
Table 1.
Baseline characteristics of the study population
| Variable | TIKI study (N = 179) |
|---|---|
| Randomized to IVIg, n (%) | 96 (53.6) |
| Age, y (median [IQR]) | 4.10 [2.49, 7.62] |
| Age, n (%) | |
| 0‐1 | 7 ( 3.9) |
| >1‐7 | 123 (68.7) |
| 7‐18 | 49 (27.4) |
| Female (%) | 83 (46.4) |
| Platelet count, ×109/L | 6.00 [3.00, 9.50] |
| Buchanan score, n (%) | |
| 0 | 3 ( 1.7) |
| 1 | 27 (15.2) |
| 2 | 75 (42.1) |
| 3 | 73 (41.0) |
| Preceding infection, n (%) | 95 (53.7) |
| Leukocytes, ×109/L | 8.20 [6.50, 10.70] |
| Lymphocytes, ×109/L | 3.80 [2.70, 5.00] |
| Symptom duration, d | 3.00 [2.00, 7.00] |
| IgG MAIPA available, n (%) | 176 (98.3) |
| IgM MAIPA available, n (%) | 167 (93.3) |
Data are median [interquartile range] unless otherwise specified.
Abbreviations: MAIPA, monoclonal antibody‐immobilization of platelet antigens; TIKI, Therapy with or without Intravenous Immunoglobulins for Newly Diagnosed Immune Thrombocytopenia in Kids
We modified our routine IgG MAIPA assay to detect glycoprotein‐specific IgM antibodies because of earlier reports of IgM anti‐platelet antibodies in childhood ITP and evaluated this test in secondary cohorts. The IgM MAIPA detected anti‐GP IIb/IIIa antibodies in newly diagnosed childhood ITP (TIKI; Figure 1A). In addition to the increased signals in childhood ITP patients, we observed that a fraction of healthy adults and children with autoimmune neutropenia (AIN) also showed presence of IgM anti‐GP IIb/IIIa antibodies, whereas adult ITP patients, chronic childhood ITP from the CINKID study, or children with JIA in remission showed this to a much lesser extent (Figure 1A). For evaluation of glycoproteins GP Ib/IX and GP V, we found comparable results as for GP IIb/IIIa (Figure S1A in supporting information). These data showed that IgM anti‐platelet antibodies were a common finding in newly diagnosed childhood ITP, but they were also present in some individuals without ITP.
Figure 1.

Glycoprotein‐specific anti‐platelet antibodies as assessed by monoclonal antibody‐specific immobilization of platelet antigens assay (MAIPA). A, Overview of anti‐glycoprotein (GP) IIb/IIIa specific IgM antibodies in various cohorts. Exact P‐values for a post‐hoc Nemenyi test in comparison to newly diagnosed childhood ITP (Therapy with or without Intravenous Immunoglobulins for Newly Diagnosed Immune Thrombocytopenia in Kids [TIKI] trial; N = 167) are: Healthy controls (N = 65; P = 3.2 × 10−9), Adult ITP (N = 42; P = 5.4 × 10−14), juvenile idiopathic arthritis (JIA; N = 22; P = 5.5 × 10−4); autoimmune neutropenia (AIN; N = 48; P = 3.9 × 10−4); Chronic childhood ITP (CINKID; N = 33; P = 5.4 × 10−14). B, Overview of anti‐GP IIb/IIIa specific IgG antibodies in various cohorts. Exact P‐values for a post‐hoc Nemenyi test in comparison to newly diagnosed childhood ITP (TIKI; N = 176) are: Healthy controls (N = 68; P = 3.8 × 10−4), JIA (N = 11; P = 5.9 × 10−3); Chronic childhood ITP (CINKID; N = 33; P = .30). C, IgM anti‐platelet glycoprotein‐specific antibody levels in newly diagnosed childhood ITP (TIKI). D, IgG anti‐platelet glycoprotein‐specific antibody levels in newly diagnosed childhood ITP (TIKI). E, Venn diagrams of overlap of positive anti‐platelet glycoprotein antibodies within single patients for IgM (left panel) and IgG (right panel). F, Principal component analysis of all glycoprotein‐specific antibody levels reveals reduction of IgM and IgG antibody levels in two distinct vectors. PC1 and PC2 described ~80% of the total variance. Values were log10 transformed before calculating eigenvalues to account for skewedness of the data. G, Correlation between glycoprotein‐specific antibody levels. In panels A‐C, the dashed line (optical density [OD] 0.130) is showing the cut‐off of positive and negative results
We next assessed the prevalence of anti‐platelet IgG. Anti‐GP IIb/IIIa antibodies were present at a low frequency among children with ITP (CINIKID and TIKI). A few positive test results were present amongst children with JIA in remission or healthy controls (Figure 1B). As above, we found comparable results for glycoproteins GP Ib/IX and GP V (Figure S1B).
Among the children with newly diagnosed ITP, 103/167 (62%) had circulating IgM anti‐platelet antibodies to either GP IIb/IIIa, GP Ib/IX, or GP V (Figure 1C). In contrast, 10% of children (17/176) showed IgG antibodies to any of the three glycoproteins (Figure 1D). Of these 17 patients with IgG anti‐platelet antibodies, 5/15 patients with available test results also showed IgM antibodies. We further observed that the majority of patients who tested positive with GP‐specific antibodies regularly showed a positive test to another GP‐specific antibody of the same class (Figure 1E), but without cross‐over of IgM and IgG antibodies (Table S1 in supporting information). Principal component analysis of the multidimensional results of the six anti‐glycoprotein antibody tests (each test is one vector) showed that the results of a patient could be summarized along two distinct projections for IgG and IgM (Figure 1F). This was also reflected by a high between‐test correlation between the IgG and IgM tests, but limited correlation between IgG and IgM tests (Figure 1G; numerical values in Table S2 in supporting information). Thus, a patient with a positive IgG anti‐platelet antibody did not necessarily show an IgM response for the same glycoprotein, and vice versa.
In summary, the dominant circulating anti‐platelet antibody class detected in childhood ITP was anti‐platelet IgM. The observed responses were detected as a distinct IgM or IgG anti‐platelet antibody, with partial overlap, and directed against multiple glycoproteins.
3.2. Circulating IgM anti‐platelet antibodies are associated with young age and immune activation
We next investigated if circulating IgG or IgM anti‐platelet antibodies were associated with clinical characteristics among the children with newly diagnosed ITP. The children with IgM anti‐platelet antibodies were younger (Figure 2A; P < .001), showed prolonged duration of symptoms (Figure 2B; P = .009), were more often male (Figure 2C; P = .006), and had higher leukocyte and lymphocyte counts (P < .001; P = .008). Of the patients with a preceding infection, 62% (55/89) had detectable IgM anti‐platelet antibodies, compared to 62% (47/76) in the group without a preceding infection (P = 1.0). We found no association with the presence of the human Fc‐gamma receptor FCGR2C open reading frame (ORF) variant (P = .81), which is associated with transient ITP.36 Thrombopoietin (TPO) levels were similar between children with and without IgM anti‐platelet antibodies (median [interquartile range (IQR)]; 37 [24‐72] versus 38 [27‐54] IU/mL, respectively; P = .73). There were no detectable differences in clinical characteristics of the small group of patients with IgG anti‐platelet antibodies, compared to patients without. Like for IgM platelet antibodies, no differences in circulating TPO levels were observed between IgG anti‐platelet antibody‐positive and antibody‐negative patients (median [IQR]; 33 [23‐68] versus 38 [26‐60] IU/mL, respectively; P = .47). Taken together, these data suggested that children with IgM anti‐platelet antibodies might represent a subgroup amongst a spectrum of clinically diagnosed ITP.
Figure 2.

Clinical characteristics of patients with positive IgM anti‐platelet antibodies. A, Children with circulating IgM antibodies were younger (P < .001); B, showed longer duration of symptoms (P = .009); C, and were more often male (P = .006)
In terms of observed bleeding at diagnosis, five children with isolated IgG anti‐GPIIb/IIIa antibodies showed no more severe bleeding tendency than children without IgG anti‐platelet antibodies (Buchanan scores of 1, 1, 2, 2, 3). In the whole cohort, 17% of children exhibited mild bleeding (Buchanan score ≤2). Compared to this, mild bleeding was observed in 1/28 (4%) of children with isolated IgM anti‐GPIIb/IIIa antibodies, and 14/62 (20%) without any IgM autoantibody (Fisher's exact test; P = .03).
3.3. Assessment of the anti‐platelet antibody response during the course of disease and treatment
Next, the presence and evolution of platelet antibodies was evaluated during 1‐year follow‐up. We first assessed if IgM anti‐platelet antibody responses were stable over time. Strikingly, the anti‐GP IIb/IIIa IgM anti‐platelet antibody response was short‐lived, as samples obtained 1 week after diagnosis showed no more anti‐platelet antibodies (Figure 3A). At this time point, the majority of patients in the observation group were still thrombocytopenic, suggesting that thrombocytopenia is maintained by mechanisms which are not detected by this assay. In addition, 1 year after diagnosis, we observed no new formation of IgM anti‐platelet antibodies. This suggested that IgM anti‐platelet antibodies mark a biological phenomenon that is present in a confined time period around the diagnosis of ITP, where delayed assessment of IgM anti‐platelet antibodies may miss early antibody responses.
Figure 3.
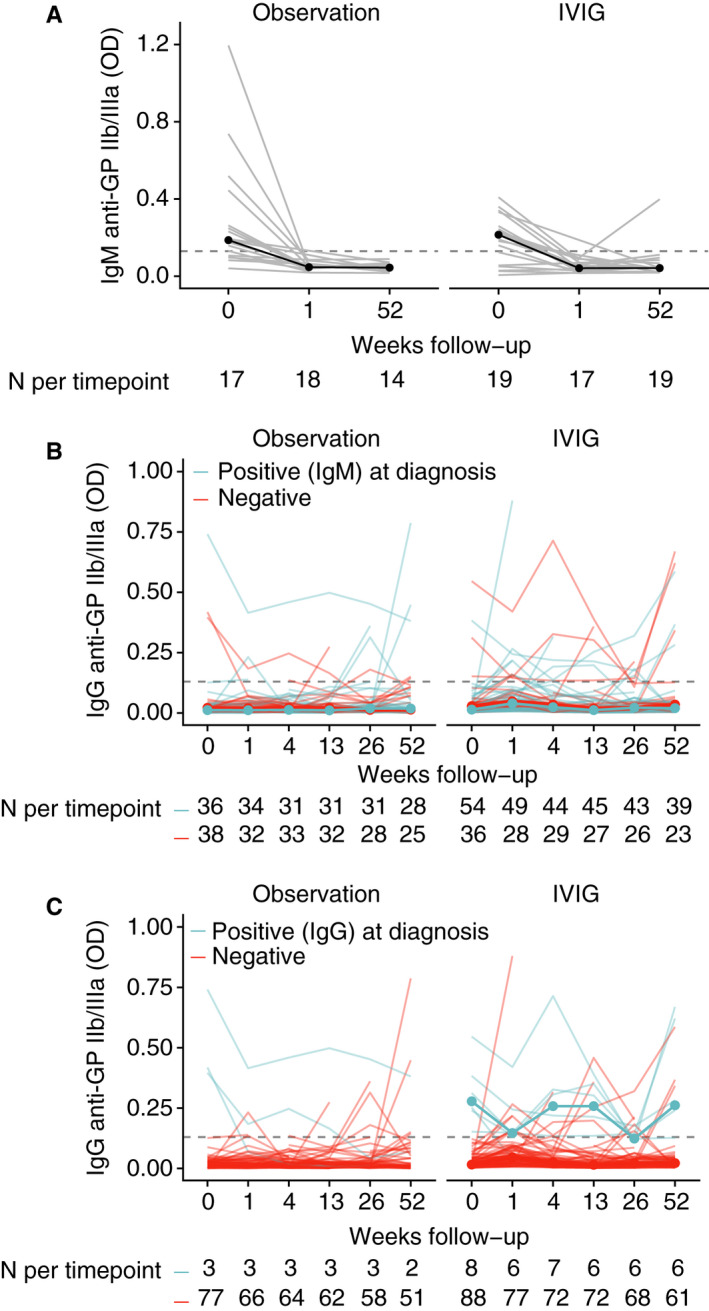
Glycoprotein‐specific anti‐platelet antibody measurements during 1‐year follow‐up. A, Longitudinal measurement of IgM anti‐GP IIb/IIIa antibody levels revealed disappearance of the antibodies already 1 week after diagnosis and persistent negative results 1 year thereafter. B, Patients who showed IgM anti‐GP IIb/IIIa antibodies did not class‐switch to IgG anti‐GP IIb/IIIa antibodies during follow‐up. C, The majority of patients with positive IgG antibody tests kept an anti‐GP IIb/IIIa antibody during 1‐year follow‐up. In all panels the dashed line indicates the optical density 0.130, the cut‐off of positive and negative results according to technical and healthy controls
A key question was if patients with IgM antibodies at diagnosis would show class‐switching to IgG antibodies during follow‐up. Interestingly, we found no evidence of a preferential occurrence of new circulating anti‐GP IIb/IIIa antibodies in patients with IgM anti‐platelet antibodies at diagnosis for the same antigen (Figure 3B) for both treated and untreated patients.
Finally, of the patients who initially showed an IgG anti‐GP IIb/IIIa antibody, ~50% kept this antibody response over at least 1 year (Figure 3C), even after the patients showed resolution of thrombocytopenia and recovered from ITP. A small proportion of patients showed de novo increases of anti‐GP IIb/IIIa antibody levels during follow‐up (Figure 3C). Amongst the treated patients, 1 week after IVIg administration there was a statistically significant increase in anti‐platelet IgG responses against all three assessed glycoproteins (data not shown), which was not shown in the observation group, and was lost again at 1‐month follow‐up. However, we could replicate a similar increase in MAIPA signals in vitro by incubating healthy donor platelets with IVIg (data not shown).
Together, these data indicated that the IgM anti‐platelet antibody response was present only short term and downregulated after 1 week, even when no treatment was given and many patients were still thrombocytopenic. There was no observable class‐switching. On the other hand, a large proportion of IgG anti‐platelet antibody responses were present long term, even after resolution of thrombocytopenia, suggesting differential effector functions of these antibodies.
3.4. Presence of anti‐platelet antibodies is associated with favorable disease courses
Finally, we assessed the prognostic significance of anti‐platelet antibodies. After 1 month follow‐up was reached, we observed that patients with IgM anti‐platelet antibodies at diagnosis in the observation cohort recovered faster than patients without (Figure 4A,B). This difference remained statistically significant (mixed effects model; P = 7.1 × 10−5) when we performed multivariate analyses with age and preceding infections as covariates, indicating that IgM anti‐platelet antibodies independently determined prognosis in this group. In contrast, IVIg‐treated patients positive for IgM anti‐platelet antibodies at diagnosis showed no statistically significant differences in platelet count or response rates during follow‐up (Figure 4A).
Figure 4.

Prognostic significance of IgM and IgG anti‐platelet antibodies. A, Patients with IgM anti‐platelet antibodies against GP IIb/IIIa showed higher platelet counts during spontaneous recovery after 1‐month follow‐up. P‐value for mixed effects model with random effects for patient and fixed effects interaction between time and IgM antibody status. B, Complete recovery (CR) rate based on IgM antibody status. No effect was observed for IVIg‐treated patients, as shown in (A). No significance test was performed due to the crossing survival curves at week 1 (nd, not determined). C, Patients with anti‐platelet IgG antibodies showed higher platelet counts after IVIg. P‐value for mixed effects model with random effects for patient and fixed effects interaction between time and IgM antibody status. D, CR rate based on IgG antibody status. Patients with IgG anti‐platelet antibodies showed complete recovery after IVIg treatment. P‐value for a log‐rank test. For the observation group no significance test was performed due to the crossing survival curves (nd, not determined)
With regard to IgG anti‐platelet antibodies, only few patients showed positive test results at diagnosis. We did not test differences in the observation group due to low sample numbers (Figure 4C). In IVIg‐treated patients, we observed that patients with IgG anti‐platelet antibodies showed increased platelet counts over time and a very high complete response rate to IVIg, much higher than the ~70% average observed amongst the whole group randomized to IVIg treatment31 (Figure 4C,D). Although the IgG antibody‐positive group was small, this finding was statistically significant. The most prevalent IgG anti‐platelet antibody was anti‐GP IIb/IIIa, and focusing only on this group of patients showed the same result.
These data indicate that patients with IgM anti‐platelet antibodies had a more favorable natural course of disease, compared to patients without. The data further suggested that when IgG anti‐platelet antibodies were present, a favorable response to IVIg could be expected.
4. DISCUSSION
4.1. Main findings
We describe the presence, longitudinal evolution, and prognostic significance of antigen‐specific platelet antibodies in newly diagnosed immune thrombocytopenia. The key findings of our study are that circulating anti‐platelet antibodies are predominantly of the IgM class, and these antibody responses are present short term, without evidence of class‐switching to IgG. Detectable anti‐platelet IgG responses are apparently rare and occur separately from IgM anti‐platelet antibody responses. No increases in circulating TPO levels among the antibody‐positive patients were observed, suggesting that megakaryocytopoiesis is not supressed.37 The presence of IgM anti‐platelet antibodies is associated with a favorable spontaneous recovery from disease, also after taking into account age and preceding infection status. The patients with IgG anti‐platelet antibodies against one of the major platelet glycoprotein complexes show higher longitudinal platelet counts and complete response rates after IVIg treatment. Finally, patients with IgM anti‐GP IIb/IIIa antibodies exhibited more severe bleeding, suggesting functional impairment of platelet aggregation. Notably, this observation was made on a subset of patients and should be further investigated. Altogether, this modern reassessment of glycoprotein‐specific antibody testing in a well‐characterized and longitudinally followed patient population provides answers to the long‐standing debate pertaining to the relevance of platelet anti‐platelet antibody testing in newly diagnosed childhood ITP.
4.2. Findings of others
In a systematic review, we have identified 40 studies that investigated direct and indirect tests to detect anti‐platelet antibodies in childhood ITP.38 However, due to heterogeneous and selective patient populations, varying sampling times from diagnosis and inference of treatments, most studies did not allow assessment of the diagnostic or prognostic value of anti‐platelet antibody assessment. The studies that allowed assessment of sensitivity and specificity against healthy controls and non‐immune thrombocytopenic controls showed moderate sensitivity and good specificity, indicating that antibodies were present in a proportion of childhood ITP patients (Schmidt et al; in press). These findings are in agreement with our results. Moreover, our finding of a high proportion of IgM anti‐platelet antibodies by a newly developed glycoprotein‐specific IgM MAIPA agrees with and extends data from three previous studies that assessed IgM anti‐platelet antibodies, but not in an antigen‐specific manner.13, 14, 15
In contrast to our study, Biglino et al27 assessed glycoprotein‐specific IgG antibodies in 74 children with transient or chronic disease courses and found no association with prognosis. Remarkably, the prevalence of circulating IgG antibodies in their study was above 30%, indicating differences in the patient population (selection bias) or technical performance of MAIPA.
One recent study in 134 Chinese children with ITP has suggested that isolated anti‐GP Ib/IX IgG antibodies could be associated with chronic ITP.28 However, the observed predictive association of isolated anti‐GP Ib/IX antibodies with chronic disease was based on only four patients. Moreover, the association did not persist when both anti‐GP IIb/IIIa and anti‐GP Ib/IX antibodies were present, in contrast to data observed in adult ITP.39, 40 Importantly, our data show that antigen‐specific antibodies in childhood ITP do not occur isolated from, but responses for the different platelet antigens correlate strongly with, distinct IgG and IgM response patterns (Figure 1). The presence of multiple platelet antibodies at the same time could indicate epitope spreading in childhood ITP, and provides serological evidence for a previously described correlation between anti‐GPIIb/IIIa and anti‐GPIb IgG producing B cells in circulation in ITP.41 While we interpret this phenomenon as the presence of antibodies against multiple antigens, it remains possible that platelet antibodies of ITP patients have a different avidity or propensity to complex formation or promotion of macromolecular interactions toward co‐precipitation.
The serological presence of IgM anti‐platelet antibodies may implicate a role for complement‐mediated clearance of platelets, which has been suggested in immune thrombocytopenia.42, 43 We could not show this experimentally when we tested a group of sera on healthy human donor platelets (data not shown).
A proposed working mechanism of IVIg is the clearance of platelet anti‐platelet antibodies through saturation of the human neonatal Fc receptor (FcRn).44, 45 The low number of patients with detectable circulating IgG anti‐platelet antibodies in our study made it impossible to test this hypothesis. However, in the long term, treatment with IVIg did not completely abolish anti‐platelet antibodies; in fact, we could detect IgG anti‐platelet antibodies for at least 1 year in about half of patients with platelet antibodies at diagnosis. This is in full agreement with the antigen‐specific assessment of anti‐platelet IgG antibodies in patients with ITP in the past, from which they have recovered.46 In adult ITP patients, the response rate to IVIg has been suggested to be reduced when GPIb‐specific IgG anti‐platelet antibodies39, 40 or anti‐GPIb IgG producing B cells are found in circulation.41 Moreover, the response rate to rituximab was reduced in adult ITP patients without IgG anti‐platelet antibodies47, 48 although conflicting data have been published.49 Our data indicate that amongst children with ITP, the absence of detectable IgG anti‐platelet antibodies is associated with lower response rates to IVIg, compared to children who do show IgG anti‐platelet antibodies.
4.3. Strengths and limitations
An important limitation of circulating anti‐platelet antibody testing in autoimmune thrombocytopenia is the problem that antibodies with a high affinity and excellent effector functions are likely to have bound to platelets and may have been cleared preferentially in vivo. This is emphasized by a generally higher sensitivity of direct cell‐bound anti‐platelet antibody tests in ITP.11, 26, 50, 51 In juvenile patients, due to the limited sample volume and the low platelet counts in ITP, we were not able to perform direct anti‐platelet antibody tests on patients’ cells. Nonetheless, we show that indirect platelet antibody testing can still be of clinical benefit, as results correlated with clinical follow‐up.
Many previous publications calculated sensitivity and specificity of anti‐platelet antibody tests with the clinical diagnosis of ITP as gold standard (reference). With the known heterogeneity due to case‐mix of the clinical diagnosis, and the absence of a reference group of children investigated for suspected ITP, such accuracy estimates can be misleading. A main strength of our study to circumvent this problem was to perform analyses directly related to longitudinal patient platelet counts and clinical response.
4.4. Implications
Despite a long history of research, the role of antiplatelet antibody testing in childhood ITP remains under debate.29, 30 Our study suggests several clinical implications. First, the assessment of circulating glycoprotein‐specific anti‐platelet antibodies may be helpful in determining the chance of spontaneous recovery. Second, the presence of IgG glycoprotein‐specific anti‐platelet antibodies was significantly associated with a complete response to IVIg. In such patients, treatment could be considered with a high chance of response, and IVIg treatment may be a useful rescue therapy in case of severe bleeding. These results may aid in the clinical counseling of patients and their families. Importantly, the study's results should be confirmed in a secondary validation study before clinical implementation.
Because of the potential ongoing clearance of anti‐platelet antibodies in vivo, it is possible that the detection of circulating anti‐platelet IgG antibodies reflect merely the “tip of an iceberg.” We suggest that more sensitive (direct) laboratory techniques, which can be employed with low cell numbers, should be evaluated in respect to prognosis in childhood ITP. For instance, a direct MAIPA has a sensitivity of 80% to 90% in adult ITP,11, 52 but children often have insufficient platelets available for this assay. Surface‐plasmon resonance (SPR), which measures antigen‐antibody interactions on a chip, can detect circulating low‐avidity platelet antibodies that would be missed in an indirect MAIPA,10 but currently not all relevant antigens can be immobilized to the chip. Cellular SPR, in which sensitized cells are perfused over a microchip coated with Fc receptors that detect cell‐bound antibodies,53, 54 could be a sensitive alternative that should be explored for ITP.
5. CONCLUSIONS
In newly diagnosed childhood ITP, glycoprotein‐specific anti‐platelet antibody testing may be useful to determine the prognosis and potential response to IVIg. IgM anti‐platelet antibodies are the dominant circulating antibody class. IgM antibodies are short‐lived, without evidence of class‐switching to IgG antibodies. Finally, our data suggest more sensitive techniques should be developed and evaluated to determine anti‐platelet antibodies for prognosis in childhood ITP.
CONFLICTS OF INTEREST
DES, KMJHP, MCAB, LP, CEvdS, GV, and MdH declare no competing financial interests.
AUTHOR CONTRIBUTIONS
DES coordinated laboratory analyses, analyzed and interpreted data, and wrote the manuscript. KMJHP and MCAB designed the clinical studies, collected data, and discussed results. LP coordinated and supervised laboratory analyses. CEvdS and GV interpreted and discussed data. MdH designed and supervised the study. All co‐authors reviewed, revised, and approved the manuscript.
Supporting information
Supinfo
ACKNOWLEDGMENTS
This study would not have been possible without the dedication of the staff of the Laboratory for Platelet and Granulocyte Serology at Sanquin, specifically Elly Huiskes, Gonda Oldert, Ilona Comijs, Laura Onderwater‐van den Hoogen, Marrie Dirkson, Roxanne Kortekaas, Suzanne Hofstede‐van Egmond, and Tarja Kochx.
Schmidt DE, Heitink‐Polle KMJ, Porcelijn L, et al. Anti‐platelet antibodies in childhood immune thrombocytopenia: Prevalence and prognostic implications. J Thromb Haemost. 2020;18:1210–1220. 10.1111/jth.14762
Manuscript handled by: Andreas Greinacher
Final decision: Andreas Greinacher, 11 February 2020
Funding information
This work was supported by a research grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR) and a doctoral stipend to DES by the Studienstiftung des Deutschen Volkes.
Contributor Information
David E. Schmidt, @schmidtdav.
Masja de Haas, Email: m.dehaas@sanquin.nl.
REFERENCES
- 1. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. [DOI] [PubMed] [Google Scholar]
- 3. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511‐6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vanderlugt CL, Miller SD. Epitope spreading in immune‐mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2(2):85‐95. [DOI] [PubMed] [Google Scholar]
- 5. Heitink‐Polle KMJ, Nijsten J, Boonacker CWB, de Haas M, Bruin MCA. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta‐analysis. Blood. 2014;124(22):3295‐3307. [DOI] [PubMed] [Google Scholar]
- 6. Cines DB, Cuker A, Semple JW. Pathogenesis of immune thrombocytopenia. Presse Med. 2014;43(4 Pt 2):e49‐e59. [DOI] [PubMed] [Google Scholar]
- 7. Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38(1):1‐10. [PubMed] [Google Scholar]
- 8. Shulman NR, Marder VJ, Weinrach RS. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann NY Acad Sci. 1965;124(2):499‐542. [DOI] [PubMed] [Google Scholar]
- 9. van Leeuwen EF, van der Ven JT, Engelfriet CP, von dem Borne AE. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood. 1982;59(1):23‐26. [PubMed] [Google Scholar]
- 10. Vollenberg R, Jouni R, Norris PAA, et al. Glycoprotein V is a relevant immune target in patients with immune thrombocytopenia. Haematologica. 2019;104:1237‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Porcelijn L, Huiskes E, Oldert G, Schipperus M, Zwaginga JJ, de Haas M. Detection of platelet autoantibodies to identify immune thrombocytopenia: state of the art. Br J Haematol. 2018;39(Suppl. 1):195. [DOI] [PubMed] [Google Scholar]
- 12. van Leeuwen EF, von dem Borne AE, van der Plas‐van DC, Engelfriet CP. Idiopathic thrombocytopenic purpura in children; detection of platelet autoantibodies by immunofluorescence. Scand J Haematol. 1981;26(4):285‐291. [DOI] [PubMed] [Google Scholar]
- 13. Nielsen OH, Tuckuviene R, Nielsen KR, Rosthøj S. Flow cytometric measurement of platelet‐associated immunoglobulin in children with newly diagnosed immune thrombocytopenia. Eur J Haematol. 2016;96(4):397‐403. [DOI] [PubMed] [Google Scholar]
- 14. Iyori H, Fujisawa K, Akatsuka J. Autoantibodies and CD5+ B cells in childhood onset immune thrombocytopenic purpura. Acta Paediatr Jpn. 1995;37(3):325‐330. [DOI] [PubMed] [Google Scholar]
- 15. Winiarski J. IgG and IgM antibodies to platelet membrane glycoprotein antigens in acute childhood idiopathic thrombocytopenic purpura. Br J Haematol. 1989;73(1):88‐92. [DOI] [PubMed] [Google Scholar]
- 16. De Cuyper IM, Meinders M, van de Vijver E, et al. A novel flow cytometry‐based platelet aggregation assay. Blood. 2013;121(10):e70‐e80. [DOI] [PubMed] [Google Scholar]
- 17. Porcelijn L, Huiskes E, Maatman R, de Kreuk A, de Haas M. Acquired Glanzmann's thrombasthenia caused by glycoprotein IIb/IIIa autoantibodies of the immunoglobulin G1 (IgG1), IgG2 or IgG4 subclass: a study in six cases. Vox Sang. 2008;95(4):324‐330. [DOI] [PubMed] [Google Scholar]
- 18. Xu M, Li J, Neves MAD, et al. GPIbα is required for platelet‐mediated hepatic thrombopoietin generation. Blood. 2018;132(6):622‐634. [DOI] [PubMed] [Google Scholar]
- 19. Cines DB, Schreiber AD. Immune thrombocytopenia. Use of a Coombs antiglobulin test to detect IgG and C3 on platelets. N Engl J Med. 1979;300(3):106‐111. [DOI] [PubMed] [Google Scholar]
- 20. von dem Borne AEGK, Verheugt FWA, Oosterhof F, Riesz E, Rivière AB, Engelfriet CP. A simple immunofluorescence test for the detection of platelet antibodies. Br J Haematol. 1978;39(2):195‐207. [DOI] [PubMed] [Google Scholar]
- 21. Kelton JG, Steeves K. The amount of platelet‐bound albumin parallels the amount of IgG on washed platelets from patients with immune thrombocytopenia. Blood. 1983;62(4):924‐927. [PubMed] [Google Scholar]
- 22. Mueller‐Eckhardt C, Kayser W, Mersch‐Baumert K, et al. The clinical significance of platelet‐associated IgG: a study on 298 patients with various disorders. Br J Haematol. 1980;46(1):123‐131. [DOI] [PubMed] [Google Scholar]
- 23. Warner MN, Moore JC, Warkentin TE, Santos AV, Kelton JG. A prospective study of protein‐specific assays used to investigate idiopathic thrombocytopenic purpura. Br J Haematol. 1999;104(3):442‐447. [DOI] [PubMed] [Google Scholar]
- 24. Kiefel V, Santoso S, Weisheit M, Mueller‐Eckhardt C. Monoclonal antibody–specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet‐reactive antibodies. Blood. 1987;70(6):1722‐1726. [PubMed] [Google Scholar]
- 25. Berchtold P, McMillan R, Tani P, Sommerville‐Nielsen S, Blanchette VS. Autoantibodies against platelet membrane glycoproteins in children with acute and chronic immune thrombocytopenic purpura. Blood. 1989;74(5):1600‐1602. [PubMed] [Google Scholar]
- 26. Arnold DM, Santoso S, Greinacher A, On behalf of the Platelet Immunology Scientific Subcommittee of the ISTH . Recommendations for the implementation of platelet autoantibody testing in clinical trials of immune thrombocytopenia. J Thromb Haemost. 2012;10(4):695‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biglino P, Perutelli P, Mori PG. Circulating antiplatelet antibody specificity in children with immune thrombocytopenic purpura at onset. Haematologica. 1997;82(1):127. [PubMed] [Google Scholar]
- 28. Fu L, Cheng Z, Gu H, Wu R. Platelet‐specific antibodies and differences in their expression in childhood immune thrombocytopenic purpura predicts clinical prognosis. Pediatr Investig. 2018;2(4):230‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Provan D, Stasi R, Newland AC,, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168‐186. [DOI] [PubMed] [Google Scholar]
- 30. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190‐4207. [DOI] [PubMed] [Google Scholar]
- 31. Heitink‐Pollé KMJ, Uiterwaal CSPM, Porcelijn L, et al. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: a randomized controlled trial. Blood. 2018;132(9):883‐891. [DOI] [PubMed] [Google Scholar]
- 32. van Bladel ER, Laarhoven AG, van der Heijden LB, et al. Functional platelet defects in children with severe chronic ITP as tested with 2 novel assays applicable for low platelet counts. Blood. 2014;123(10):1556‐1563. [DOI] [PubMed] [Google Scholar]
- 33. Zwaginga JJ, van der Holt B, Te Boekhorst PA, et al. Multi‐center randomized open label phase II trial on three rituximab dosing schemes in immune thrombocytopenia patients. Haematologica. 2015;100(3):e90‐e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. 2002;141(5):683‐688. [DOI] [PubMed] [Google Scholar]
- 35. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.R‐project.org/ [Google Scholar]
- 36. Schmidt DE, Heitink‐Pollé KMJ, Laarhoven AG, et al. Transient and chronic childhood immune thrombocytopenia are distinctly affected by Fc‐γ receptor polymorphisms. Blood Adv. 2019;3(13):2003‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porcelijn L, Folman CC, Bossers B, et al. The diagnostic value of thrombopoietin level measurements in thrombocytopenia. Thromb Haemost. 1998;79(6):1101‐1105. [PubMed] [Google Scholar]
- 38. Schmidt DE, Lakerveld AJ, Heitink‐Pollé KMJ, et al. Anti‐platelet antibody immunoassays in childhood immune thrombocytopenia: a systematic review. Vox Sang. 2020. 10.1111/vox.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peng J, Ma S‐H, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost. 2014;12(4):497‐504. [DOI] [PubMed] [Google Scholar]
- 40. Go RS, Johnston KL, Bruden KC. The association between platelet autoantibody specificity and response to intravenous immunoglobulin G in the treatment of patients with immune thrombocytopenia. Haematologica. 2007;92(2):283‐284. [DOI] [PubMed] [Google Scholar]
- 41. Kuwana M, Okazaki Y, Ikeda Y. Detection of circulating B cells producing anti‐GPIb autoantibodies in patients with immune thrombocytopenia. PLoS ONE. 2014;9(1):e86943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Najaoui A, Bakchoul T, Stoy J, et al. Autoantibody‐mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol. 2012;88(2):167‐174. [DOI] [PubMed] [Google Scholar]
- 43. Peerschke EIB, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148(4):638‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bleeker WK, Teeling JL, Hack CE. Accelerated autoantibody clearance by intravenous immunoglobulin therapy: studies in experimental models to determine the magnitude and time course of the effect. Blood. 2001;98(10):3136‐3142. [DOI] [PubMed] [Google Scholar]
- 45. Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13(3):176‐189. [DOI] [PubMed] [Google Scholar]
- 46. Imbach P, Tani P, Berchtold W, et al. Different forms of chronic childhood thrombocytopenic purpura defined by antiplatelet autoantibodies. J Pediatr. 1991;118(4 Pt 1):535‐539. [DOI] [PubMed] [Google Scholar]
- 47. Cooper N, Stasi R, Cunningham‐Rundles S, Cesarman E, McFarland JG, Bussel JB. Platelet‐associated antibodies, cellular immunity and FCGR3a genotype influence the response to rituximab in immune thrombocytopenia. Br J Haematol. 2012;158(4):539‐547. [DOI] [PubMed] [Google Scholar]
- 48. Porcelijn L, Huiskes E, Schipperus M, van der Holt B, de Haas M, Zwaginga JJ. Lack of detectable platelet autoantibodies is correlated with non‐responsiveness to rituximab treatment in ITP patients. Blood. 2017;129:3389‐3391. [DOI] [PubMed] [Google Scholar]
- 49. Arnold DM, Vrbensky JR, Karim N, et al. The effect of rituximab on anti‐platelet autoantibody levels in patients with immune thrombocytopenia. Br J Haematol. 2017;76(Suppl 1):205‐206. [DOI] [PubMed] [Google Scholar]
- 50. Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen‐specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88(1):194‐201. [PubMed] [Google Scholar]
- 51. Kiefel V, Freitag E, Kroll H, Santoso S, Mueller‐Eckhardt C. Platelet autoantibodies (IgG, IgM, IgA) against glycoproteins IIb/IIIa and Ib/IX in patients with thrombocytopenia. Ann Hematol. 1996;72(4):280‐285. [DOI] [PubMed] [Google Scholar]
- 52. Al‐Samkari H, Rosovsky RP, Karp Leaf RS, et al. A modern reassessment of glycoprotein‐specific direct platelet autoantibody testing in immune thrombocytopenia. Blood Adv. 2020;4(1):9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Szittner Z, Bentlage AEH, Donk E, et al. Multiplex blood group typing by cellular surface plasmon resonance imaging. Transfusion. 2019;59(2):754‐761. [DOI] [PubMed] [Google Scholar]
- 54. Schasfoort R, Abali F, Stojanovic I, Vidarsson G, Terstappen L. Trends in SPR cytometry: advances in label‐free detection of cell parameters. Biosensors. 2018;8(4):102‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


