Figure 1.
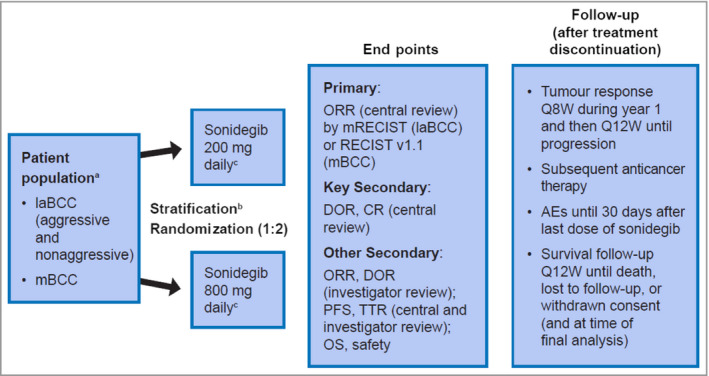
Design of the BOLT study – Basal Cell Carcinoma Outcomes with LDE225 (sonidegib) Treatment. aPatients previously treated with sonidegib or other hedgehog pathway inhibitors were excluded. bStratification was based on stage, disease histology for patients with laBCC (nonaggressive vs. aggressive) and geographical region. cTreatment was continued until disease progression, unacceptable toxicity, death, study termination or withdrawal of consent. AE, adverse event; BCC, basal cell carcinoma; CR, complete response; DOR, duration of response; laBCC, locally advanced BCC; mBCC, metastatic BCC; mRECIST, modified Response Evaluation Criteria in Solid Tumors; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; Q8W, every 8 weeks; Q12W, every 12 weeks; TTR, time to tumour response.
