Figure 1.
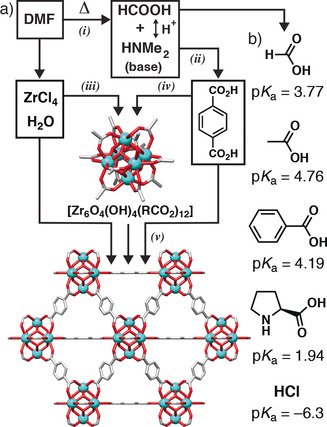
a) Schematic of variables to consider in the self‐assembly of UiO‐66: (i) the thermal decomposition of DMF to release base and formic acid, a potential coordination modulator, with associated proton balance (temperature); (ii) the deprotonation of the 1,4‐benzenedicarboxylate (BDC) linker (pH); (iii) the formation of the hexanuclear [Zr6O4(OH)4(RCO2)12] secondary building unit (Zr source, water content); (iv) the level of incorporation of the BDC linker in these SBUs and competition from additional ligands (modulators); (v) final substitution of transient ligands/modulators to allow coalescence into the UiO‐66 structure, redrawn from CCDC deposition RUBTAK,16 and its subsequent nucleation (reagent concentration). b) Typical coordination modulators used in the self‐assembly of Zr MOFs.
