Figure 1.
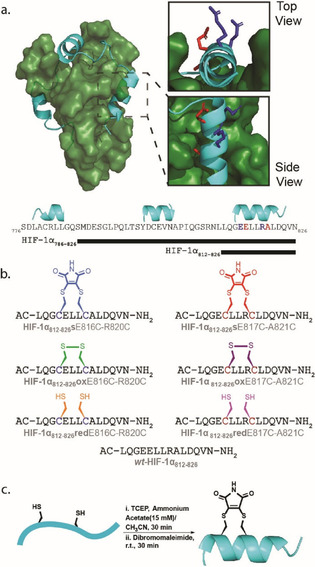
Design and synthesis of constrained HIF‐1α peptides as HIF‐1α/p300 inhibitors; a) HIF‐1α/p300 NMR solution structure (PDB:1L8C, p300 in forest green, HIF‐1α in cyan) with expansion (right) illustrating helix 3 (residues 812–826) and residues which were substituted for cysteine and subjected to stapling using dibromomaleimide, b) primary structure of the two HIF‐1α812–826 sE816C‐R820C and HIF‐1α812–826 sE817C‐A821C dibromomaleimide stapled variants: primary structures of dibromomaleimide (s), Oxidised (ox) and reduced (red) together with wild‐type (wt) sequence wt‐ HIF‐1α812–826, c) generic reaction scheme for preparation of dibromomaleimide stapled peptides and idealised helical conformation adopted as a consequence of stapling.
