Figure 1.
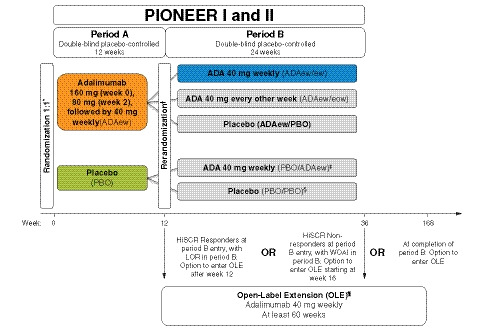
PIONEER study schematic. *Stratified by baseline Hurley Stage II vs. III (PIONEER I & II) and baseline concomitant antibiotic use (PIONEER II). Rerandomization at entry to period B, stratified by week 12 HiSCR status and BL Hurley Stage II vs. III. ‡PIONEER I only (160 mg week 12, 80 mg week 14, 40 mg from week 16). §PIONEER II only. ¶OLE entry criteria: completed PIONEER I or II or met prespecified escape criteria (LOR or WOAI in period B). LOR was defined as a count greater than the average AN count at baseline and at week 12. Patients who achieved HiSCR at week 12 and experienced LOR during period B were discontinued from the study and were eligible to enter the OLE and receive open‐label ADAew. WOAI was defined as AN count greater than or equal to baseline AN count at two consecutive visits (excluding week 12) occurring ≥ 14 days apart. Patients who did not achieve HiSCR at week 12 continued in period B through at least week 16 (and up to week 36). At or after week 16, patients who experienced WOAI were discontinued from the study and were eligible to enter the OLE study to receive open‐label ADAew. ADA, adalimumab; AN, sum of inflammatory nodules and abscesses; BL, baseline; ew, every week; eow, every other week; HiSCR, hidradenitis suppurativa clinical response; LOR, loss of response; OLE, open‐label extension; PBO, placebo; WOAI, worsening or absence of improvement.
