Abstract
Background
Temporary disruption of sensory input can be studied relatively easily for vision or hearing by covering the eyes or ears. In contrast, closing the nostrils affects not only the sense of smell, but also the ability to breathe through the nose and humidify and warm inhaled air. We hypothesized that filling the olfactory cleft (OC) with dissolvable nasal dressing (foam) would temporarily block olfaction while respecting nasal airflow.
Methods
In 30 healthy volunteers, the OC was unilaterally obstructed in a back‐to‐front fashion. Orthonasal and retronasal olfactory function were tested before and after foam application. Ratings of odors and subjective nasal patency (SNP) were collected. Peak nasal inspiratory flow (PNIF) was used to measure nasal patency.
Results
Foam was safely applied in every case using minimal instruments. No complications were reported. Orthonasal and retronasal test results decreased significantly in overall participants (all p < 0.0008). Indicating temporary anosmia, 3 subjects reached the lowest possible score for odor‐threshold testing, with corresponding drops in retronasal test scores. PNIF values before and after foam application were not significantly different (p = 0.11). SNP ratings decreased slightly, but not significantly (p = 0.052). Odor‐intensity ratings dropped significantly (all p < 0.05).
Conclusion
The OC can be safely obstructed with dissolvable nasal dressing, resulting in a decrease in odor‐intensity and orthonasal and retronasal olfactory function test scores. This procedure may serve as a hyposmia model that maintains normal nasal airflow.
Keywords: anosmia, candies, flavor, obstruction, olfactory cleft, smell
The sense of smell contributes to flavor perception (through the retronasal route) and hence to food enjoyment. 1 Understandably, losing this valuable ability affects one's quality of life. 2
The consequences of temporarily disrupting sensory input can be studied relatively easily for vision or hearing by covering the eyes or ears. The olfactory system, however, is embedded within the nose, which is also crucial in breathing, including humidifying and warming inhaled air. Therefore, simply closing the nostrils also affects other systems. Without nasal airflow, retronasal olfactory perception is significantly diminished. 3
Attempts to temporarily switch off the sense of smell have been made. Welge‐Luessen and colleagues applied 1.5 to 2 mL of 4% lidocaine to both olfactory clefts (OCs) for 10 minutes, with the subject in a head‐down position. They observed a transient anosmia for up to 2 hours. However, only orthonasal olfactory function was measured and 4 of 10 subjects experienced side effects (headaches and nose blockage) and prolonged anosmia. 4 Pfaar et al. 5 obstructed the anterior region of the OC with sponges and found orthonasal olfactory function to be affected more strongly than retronasal olfactory function.
To date, no suitable strategy has been proposed to serve as a hyposmia/anosmia model that modifies orthonasal and retronasal odor perception while respecting nasal airflow. We hypothesized that filling the OC with dissolvable nasal dressing (which is frequently used in sinus surgery) would allow such temporary blockage of the sense of smell. The current study therefore aimed to evaluate: (1) the feasibility of OC obstruction using dissolvable nasal dressing; (2) the impact of this procedure on orthonasal and retronasal olfactory perception; and (3) its impact on nasal patency.
Subjects and methods
The protocol was approved by the ethics committee of the Medical University of Vienna (EK No. 1935/2018) and the study was conducted according to the guidelines of the Declaration of Helsinki on Biomedical Research Involving Human Subjects. All tests took place between August and November 2019. All subjects provided written informed consent prior to participation. According to article 40 of the Medical Devices Act (regulating studies including medical devices), the Austrian Federal Office for Safety in Health Care (BASG) was notified and approval was granted (Reference No. 12091001). Internal study monitoring was maintained as required.
Subjects
The study included 30 healthy volunteers (18 females, 12 males; mean ± standard deviation [SD] age, 30.7 ± 12.4 years; range, 20 to 59 years) with subjectively normal olfactory function. Subjects were recruited using flyers distributed across the university campus. Exclusion criteria were history of chronic sinusitis, previous extensive nasal/sinus surgeries, pronounced septal deviations (neither olfactory cleft accessible), and current pregnancy or breastfeeding. Of the participants, 2 stated they were smokers, 1 that they occasionally smoked, and 6 were former smokers.
Study sequence and randomization
For a flowchart of the study protocol, see Figure 1. Olfactory tests were performed before and after foam application. To investigate whether foam application was feasible with simple instruments (a nasal speculum or endoscope), participants were randomly assigned to an endoscopy (Endo) or a nasal speculum (Spec) group following a preprogrammed randomization list (the first randomization list). The nasal side allowing easiest access to the OC region was chosen for foam application. In case of a straight nasal septum, the side was assigned randomly based on a second randomization list.
FIGURE 1.
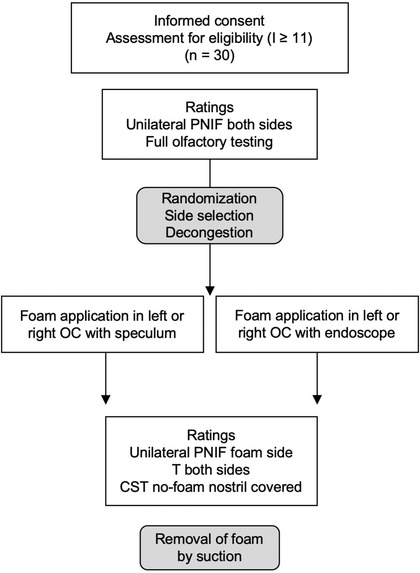
Study profile. CST = candy smell test; I = odor identification; OC = olfactory cleft; PNIF = peak nasal inspiratory flow; T = odor threshold.
OC obstruction
Foam application to the OC was performed by 2 otorhinolaryngologists (G.B. and D.T.L.) with sinus surgery experience. For local anesthesia, swabs soaked with lidocaine and epinephrine were squeezed to eliminate excess liquid, placed in the anterior nasal cavity, and left in place for 2 minutes. Placement was not close to the OC and was solely intended to facilitate decongestion and minimize irritation of the lower nasal regions. Next, according to grouping, either an endoscope or a speculum and headlight were used to visualize the entrance to the OC.
Rapid Rhino Sinu‐Foam nasal dressing (ArthroCare Corporation, Austin, TX) is a certified class 1 sterile medical device (EU Device Class) based on carboxymethylcellulose, which dissolves gradually through normal outflow within 7 to 10 days. It was used according to the manufacturer's instructions with sterile water and the provided syringe. The foam was applied to the OC in a back‐to‐front fashion (see Fig. 2) with the aim of efficiently obstructing both the orthonasal and retronasal routes. The second olfactory testing started approximately 15 minutes later. Following this, the foam was removed by suction. The duration of the entire procedure was approximately 2 hours.
FIGURE 2.
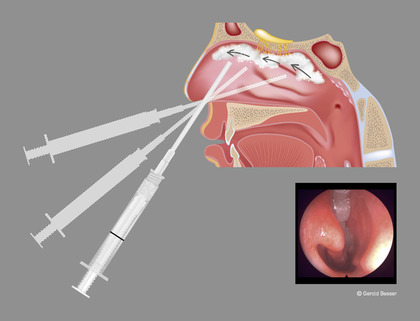
Illustration of foam application to the olfactory cleft using the provided syringe in a back‐to‐front fashion in order to also affect retronasal olfactory abilities. (Small picture) Endonasal view after foam application with a 25‐degree‐angle rigid endoscope. The inferior meatus remains free of foam. This particular subject's odor‐threshold testing score dropped from 6.25 to 1.5, but there was no change in the retronasal test score. The investigator recorded a difficult rear approach due to a septal spur. PNIF improved by 20 points following decongestion and foam application. PNIF = peak nasal inspiratory flow.
Blinding
Specialists applying the foam to the OC were not informed of the initial olfactory test results and participants were not allowed to inform the examiner of the treated side for odor‐threshold testing. This blinding approach was intended to minimize investigator (eg, choosing the better threshold side) and examiner bias (eg, quicker threshold testing after foam application due to expected anosmia). For retronasal olfactory testing (see the next section), the contralateral nostril was covered, which, in consequence, revealed the foamed side to the examiner.
Olfactory tests
All olfactory tests were performed in a well‐ventilated room by trained examiners who were not part of this investigation panel.
Orthonasal olfactory tests were performed using Sniffin’ Sticks (reusable odorant felt‐tip pens; Burghart GmbH, Wedel, Germany). First, participants were screened for olfactory dysfunction using the 16‐item odor identification test. Given the 10th percentile of this test in the 21‐to‐30‐year age group, subjects scoring below 11 points were assumed to have dysfunction and were not eligible to continue in the trial. 6 Following odor‐discrimination testing, odor threshold (using n‐butanol) was tested in a reversed‐staircase procedure for each nostril separately (the other nostril was blocked with adhesive tape, preventing nasal airflow) before and after foam application. Discrimination and identification were only tested prior to “foaming.” Detailed descriptions of these tests and large normative data sets are available elsewhere. 7 , 8 , 9 Summed scores of threshold, discrimination, and identification (TDI) help to categorize patients as normosmics (≥30.75), hyposmics (16.25 to 30.50), and anosmics (≤16). 6
Retronasal olfactory function was assessed using 27 food‐grade‐quality candies, each 9 mm in diameter and containing 500 mg sorbitol and the target aroma (the candy smell test [CST]). 10 , 11 , 12 Candies can be sucked or chewed; after each candy the mouth is rinsed with water. Answers sheets are completed in a 4‐alternative forced‐choice manner without visual cues. The maximal attainable test score is 27. For the purpose of this study, correct answers were not revealed to participants during the first testing and the order of the candies was altered for the second testing. After foam application and the second threshold testing, the foamed side was unblinded to examiners and the untreated nostril was covered with adhesive tape in order to prevent nasal air outflow.
Peak nasal inspiratory flow
A portable inspiratory flowmeter produced by Clement Clarke International Ltd. (Essex, UK) was used to measure peak nasal inspiratory flow (PNIF). The highest value of 3 satisfactory maximal inspirations (through the nose and in a sitting position) was recorded. 13 PNIF values (in L/min) were obtained separately for the left and right sides prior to swab placement (before decongestion) and, after foam application, solely for the foamed side. One nostril was sealed off using adhesive tape, as described for PNIF measurements. 14
Ratings
Prior to foam application, ratings were obtained for subjective olfactory function (SOF, from 0 mm = no sense of smell to 100 mm = best sense of smell) and subjective nasal patency (SNP) for each side (from 0 mm = total block to 100 mm = excellent patency). The specialist's confidence in foam application (SCF) was also rated on a visual analogue scale (from 0 mm = “not certain that foam occluded the OC,” to 100 mm = “very certain that OC was occluded”). After foam application, participants had to re‐rate their SNP and were asked to rate the discomfort of application (pain scale [PS]: 0 mm = no pain/discomfort to 100 mm = unbearable). Prior to retesting of olfactory function, participants rated how subjectively inconvenient the foam was in situ (SIF, from 0 mm = no inconvenience to 100 mm = very inconvenient due to blockage/pain/rhinorrhea) and were asked to state whether or not they would consider maintaining foam blockage for several days, 1 week, or 3 weeks.
Additionally, 3 selected odors from the Sniffin’ Sticks identification test and 3 corresponding candies (orange, coffee, and anise) were rated for hedonic value (from –4 = very unpleasant to 4 = very pleasant) and intensity (from 0 = nothing to 100 = very intense), before and after foaming (when after foaming, with 1 nostril covered).
Analytic plan and statistical analysis
Differences in olfactory measurements were interpreted as meaningful (in terms of C‐value 15 ) when reaching ≥2.5 points for odor threshold (adapted from Gudziol et al. 16 ) and ≥5 points for the 27‐CST (predefined by the authors, considering that the C‐value for the 16‐item orthonasal identification test was defined as 3 points by Gudziol et al., 16 and taking into account a presumable learning curve in suprathreshold identification testing regardless of the altered administration order of the candies).
IBM SPSS 26.0 (IBM Corp., Armonk, NY) and GraphPrism 8.2.0 (GraphPad Software, Inc., La Jolla, CA) were used for statistical analysis. Graphical visualization was performed using GraphPrism. Normality of data was tested using Shapiro‐Wilk test. Depending on the normality of data, group differences were tested using (paired or unpaired) sample t tests or Mann‐Whitney tests (for between‐subject variables). Data are presented as mean ± SD, as indicated. Correlational analyses were performed using the Pearson correlation coefficient (r). A p value of <0.05 was required for statistical significance.
Results
Foam application
Foam application was possible in every case; however, in 4 cases investigators stated that the posterior area was hard to reach (eg, due to a septal spur). Pain scoring was variable (41.4 ± 26.3), but all subjects tolerated the procedure and there was no need to stop (eg, due to excessive pain or bleeding) in any of the cases. Approximately 4 mL of foam was needed to fill up the OC as much as possible. The second randomization list was not utilized at all (ie, in every case 1 OC was easier to access); the right side was chosen in 20, and the left in 10 cases. Investigators were rather confident with the foam placement (SCF, 70.2 ± 24.6) and there was no significant difference in PS nor in SCF between groups Endo and Spec (all p > 0.472). SIF was rather low (15.6 ± 16.0). Only 1 subject stated the foam was too inconvenient to leave in the nose for a longer period of time, 11 subjects would leave it for several days, 6 subjects for 1 week, and 12 even, hypothetically, for 3 weeks. In 2 cases an olfactory test examiner reported they were inevitably unblinded to the foamed side because the subjects had a runny nose on that side. All subjects were instructed to contact the investigators in case of nose‐related problems arising after participation (eg, continuous discharge, pain, or blocked nose), but none did so.
Olfactory performance
None of the participants had to be excluded due to low performance on identification testing (mean I scores 13.7 ± 1.3). The overall mean TDI (best performing nostril) was 34.3 ± 3.5, with 5 subjects scoring slightly below the cutoff for normosmia. TDI and CST results correlated significantly (r 30 = 0.391, p = 0.032), similar to previous findings. 10 , 11 Interestingly, in this small group males performed significantly better on right threshold, TDI and CST than females (all p < 0.020). SOF was 67.9 ± 15.7 and not correlated with measured olfactory function (p > 0.05).
Following foam application, odor threshold decreased significantly just for the foamed side (p < 0.0001). CST scores decreased significantly over all participants (p = 0.0008). The C‐value was reached in 12 subjects for threshold and in 8 subjects for the CST. In 5 subjects (3 females, 2 males), both orthonasal (threshold after foaming: range, 1 to 3) and retronasal olfactory performance (CST difference: range, 5 to 10) were reduced to a meaningful extent, indicating a hyposmic situation in these subjects after foam application. Of these 5 subjects, 3 subjects were unable to detect the highest threshold concentrations (scoring the lowest possible score) after foam application, possibly indicating functional anosmia.
In those subjects (n = 15) experiencing a meaningful drop in olfactory performance (orthonasal, retronasal, or both) PS was not significantly higher than in those with no meaningful changes (p = 0.81), nor was SCF (p = 0.81). Table 1 shows scores, including PNIF/SNP values, before and after foam application.
TABLE 1.
Comparison of measurements before and after foam application (n = 30)
| Measurement | Before foam (mean ± SD) | After foam (mean ± SD) |
|---|---|---|
| f Threshold | 6.6 ± 2.8 a | 4.1 ± 2.9 a |
| c Threshold | 5.8 ± 2.6 | 5.7 ± 2.7 |
| CST | 20.0 ± 3.0 b | 17.6 ± 4.1 b |
| f SNP | 72.4 ± 22.1 | 60.4 ± 28.1 |
| c SNP | 69.6 ± 22.5 | 73.0 ± 18.1 |
| f PNIF (L/min) | 92.2 ± 28.8 | 84.0 ± 32.4 |
Significantly different pairs: p < 0.0001.
Significantly different pairs: p = 0.0008.
CST = candy smell test; c = contralateral side; f = foamed side; PNIF = peak nasal inspiratory flow; SNP = subjective nasal patency.
Nasal patency
PNIF values prior to foam application were comparable to published unilateral results, with lower values in females (left PNIF 90.8 ± 27.9; right PNIF 85.3 ± 23.5) than in males (left PNIF 92.9 ± 36.5; right PNIF 100.8 ± 30.5). 14 PNIF values and SNP scores for each side did not correlate significantly (all p > 0.733).
PNIF values before and after foam application were not significantly different (p = 0.11). SNP ratings decreased slightly following foam application (72.4 ± 22.1 vs 60.4 ± 28.1), but this was not significant (p = 0.052). When separating all participants into groups with meaningful olfactory changes (n = 15) and those with none, changes in PNIF and SNP values following foam application were not significantly different (PNIFdiff, p = 0.67; SNPdiff, p = 0.57).
Odor hedonic and intensity ratings
There were no gender‐specific differences in hedonic or intensity ratings (all p > 0.05). Odor ratings for hedonic value and intensity were not significantly different when the odor was presented orthonasally vs retronasally (all p > 0.174), except for the subjective intensity of anise, which was perceived to be significantly higher retronasally vs orthonasally (p = 0.0214).
Figure 3 illustrates overall ratings before and after foam application. Odor intensity dropped by 17.9 ± 35.0 for orthonasal and 11.0 ± 40.0 points for retronasal odors. The aforementioned 5 subjects with meaningful changes in orthonasal and retronasal scores showed higher mean changes in odor‐intensity ratings, specifically for the retronasal route (21.5 ± 37.8 for orthonasal odors and 36.9 ± 38.0 for retronasal odors).
FIGURE 3.
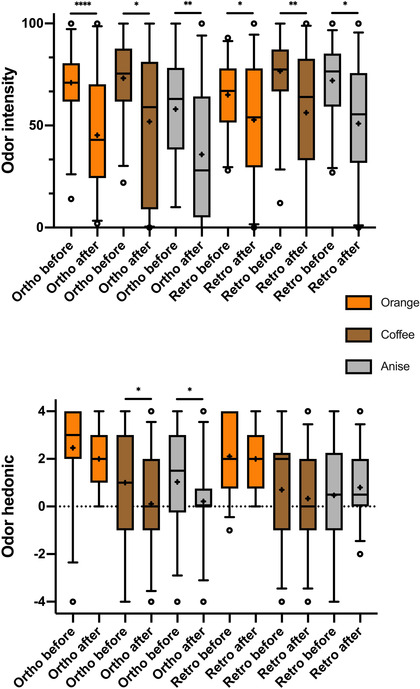
Box‐and‐whisker plots of hedonic and intensity ratings of orthonasally and retronasally presented odors before and after foam application. Odor hedonic: –4 = very unpleasant to 4 = very pleasant; odor intensity: 0 = nothing to 100 = very intense; medians (Q0.5; line), interquartile range (Q0.25, Q0.75; boxes); + indicating the mean scores. *p < 0.05, **p < 0.01, ****p < 0.0001.
Discussion
As a primary goal of this study we proved the feasibility of unilateral obstruction of the OC using dissolvable nasal dressing. The procedure was possible using minimal instruments and participants tolerated it well, as reflected by minimal pain ratings. No complications were observed. In selected cases, foam application resulted in significantly decreased orthonasal and retronasal olfactory test results while maintaining nasal airflow. This finding is emphasized by significant drops in overall odor‐intensity ratings following foam application.
Olfactory dysfunction is frequently due to chronic rhinosinusitis (CRS). In these cases, olfactory loss is due not only to changes in conduction, with odorants being blocked from reaching the OC, but also to inflammation. 17 , 18 Still, obstructive processes influence olfactory perception to a great extent in CRS, as reflected by studies on systematic scorings of edema, discharge, scarring, crusting, and polyps of the OC showing correlations with olfactory test results. 19 , 20 Also, quantifying the degree of opacification of the OC in CRS using computed tomography showed association with olfactory test results. 21 , 22 Furthermore, in CRS with nasal polyps, obstruction presumably leads to differences in orthonasal and retronasal olfactory function. 23 Therefore, it seems worthwhile to investigate obstruction models that mimic CRS and may allow novel insights into nasal diseases.
The OC, with its variable expansion, harbors the majority of the olfactory epithelium. 24 Olfactory neurons, however, can be found beyond this anatomic region, 25 which may be 1 reason that olfactory function did not decrease to the C‐value in all included subjects following foam application. Also, the foam density may have been insufficient in various areas, allowing odorants to pass this barrier. Nevertheless, the foam significantly affected orthonasal and retronasal olfactory function in one‐sixth and affected at least 1 route in one‐half of included subjects. In 3 subjects, foam application induced functional anosmia. Additionally, there were significant group differences for CST and threshold scores for the foamed but not the contralateral (control) side. These findings certainly encourage further investigation of this technique. However, 1 apparent limitation of the current study is the unilateral application of the foam: for safety reasons the study planner agreed on initial 1‐sided application and to leave dual application for future studies (analogous to the aforementioned study using sponges 5 ). However, studies attempting to block both OCs will need to select subjects with nearly straight nasal septa.
In general, odors can be perceived as pleasant or unpleasant and a shift in this subjective perception is termed parosmia (distortion of odors). In olfactory dysfunction due to CRS, however, parosmia is not common. 17 Therefore, obstruction of the OC (because the OC is often affected in CRS) may not shift the pleasantness of an odor. In order to detect changes in the pleasantness (hedonic value) and intensity of odors following OC obstruction, we applied visual analogue scales. Orthonasal and retronasal odor pairs were rated comparably in regard to pleasantness. Previous authors found a similar relation for food odors in contrast to nonfood odors. 26 Hedonic ratings did not change as pronouncedly as intensity ratings following foam application, supporting the discussed associations.
PNIF is a valuable instrument for clinicians and PNIF values were associated with subjective nasal obstruction in previous studies. 27 , 28 We found it was a suitable tool to show maintenance of nasal airflow after foam application. However, the results must be interpreted with caution because we used nasal decongestion. Available data show that PNIF values improve by 8.7 to 14.7 points following nasal decongestion. 29 , 30 Still, foam application evidently impacts nasal airflow in some individuals, but not to a very pronounced degree. Hence, this model may be useful in research on nasal patency in addition to olfaction.
Conclusion
The OC can be safely obstructed with dissolvable nasal dressing, resulting in a decrease in odor‐intensity and orthonasal and retronasal olfactory function test scores. This procedure may serve as a hyposmia model while maintaining normal nasal airflow.
Acknowledgments
We thank Dr. S. Seyferth, from the Division of Pharmaceutics at the Friedrich‐Alexander‐Universität Erlangen‐Nürnberg, Germany, for professionally manufacturing candies. Participants received 30 € as compensation for their time.
How to Cite this Article:Besser G, Liu DT, Renner B, Hummel T, Mueller CA. Reversible obstruction of the olfactory cleft: impact on olfactory perception and nasal patency. Int Forum Allergy Rhinol. 2020;10:713–718.
Funding sources for the study: Medical Scientific Fund of the Mayor of the City of Vienna MUW‐19009BGM.
Potential conflict of interest: None provided.
References
- 1. Shepherd GM. Smell images and the flavour system in the human brain. Nature. 2006;444:316‐321. [DOI] [PubMed] [Google Scholar]
- 2. Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125:116‐121. [DOI] [PubMed] [Google Scholar]
- 3. Murphy C, Cain WS. Taste and olfaction: independence vs interaction. Physiol Behav. 1980;24:601‐605. [DOI] [PubMed] [Google Scholar]
- 4. Welge‐Lüssen A, Wille C, Renner B, Kobal G. Anesthesia affects olfaction and chemosensory event‐related potentials. Clin Neurophysiol. 2004;115:1384‐1391. [DOI] [PubMed] [Google Scholar]
- 5. Pfaar O, Landis BN, Frasnelli J, Huettenbrink KB, Hummel T. Mechanical obstruction of the olfactory cleft reveals differences between orthonasal and retronasal olfactory functions. Chem Senses. 2006;31:27‐31. [DOI] [PubMed] [Google Scholar]
- 6. Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin' Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276:719‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobal G, Hummel T, Sekinger B, et al. “Sniffin' sticks”: screening of olfactory performance. Rhinology. 1996;34:222‐226. [PubMed] [Google Scholar]
- 8. Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257:205‐211. [DOI] [PubMed] [Google Scholar]
- 9. Hummel T, Kobal G, Gudziol H, Mackay‐Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237‐243. [DOI] [PubMed] [Google Scholar]
- 10. Renner B, Mueller CA, Dreier J, et al. The candy smell test: a new test for retronasal olfactory performance. Laryngoscope. 2009;119:487‐495. [DOI] [PubMed] [Google Scholar]
- 11. Haxel BR, Bertz‐Duffy S, Faldum A, et al. The Candy Smell Test in clinical routine. Am J Rhinol Allergy. 2011;25:e145‐e148. [DOI] [PubMed] [Google Scholar]
- 12. Liu DT, Besser G, Renner B, Seyferth S, Hummel T, Mueller CA. Retronasal olfactory function in patients with smell loss but subjectively normal flavor perception. Laryngoscope. 2019;57:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ottaviano G, Scadding GK, Iacono V, Scarpa B, Martini A, Lund VJ. Peak nasal inspiratory flow and peak expiratory flow. Upright and sitting values in an adult population. Rhinology. 2016;54:160‐163. [DOI] [PubMed] [Google Scholar]
- 14. Ottaviano G, Scadding GK, Scarpa B, Accordi D, Staffieri A, Lund VJ. Unilateral peak nasal inspiratory flow, normal values in adult population. Rhinology. 2012;50:386‐392. [DOI] [PubMed] [Google Scholar]
- 15. Yueh B. The threshold of clinical significance. JAMA Otolaryngol Head Neck Surg. 2020;146:98‐100. [DOI] [PubMed] [Google Scholar]
- 16. Gudziol V, Loetsch JR, Haehner A, et al. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116:1858‐1863. [DOI] [PubMed] [Google Scholar]
- 17. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 18. Soler ZM, Yoo F, Schlosser RJ, et al. Correlation of mucus inflammatory proteins and olfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;00:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soler ZM, Hyer JM, Karnezis TT, Schlosser RJ. The Olfactory Cleft Endoscopy Scale correlates with olfactory metrics in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:293‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poletti SC, Murta G, Hähner A, Hummel T. Olfactory cleft evaluation: a predictor for olfactory function in smell‐impaired patients? Eur Arch Otorhinolaryngol. 2018;275:1129‐1137. [DOI] [PubMed] [Google Scholar]
- 21. Soler ZM, Pallanch JF, Sansoni ER, et al. Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5:846‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohli P, Schlosser RJ, Storck K, Soler ZM. Olfactory cleft computed tomography analysis and olfaction in chronic rhinosinusitis. Am J Rhinol Allergy. 2016;30:402‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landis BN, Giger R, Ricchetti A, et al. Retronasal olfactory function in nasal polyposis. Laryngoscope. 2003;113:1993‐1997. [DOI] [PubMed] [Google Scholar]
- 24. Lund VJ, Stammberger H, Fokkens WJ, et al. European position paper on the anatomical terminology of the internal nose and paranasal sinuses. Rhinol Suppl. 2014;24:1‐34. [PubMed] [Google Scholar]
- 25. Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417‐421. [DOI] [PubMed] [Google Scholar]
- 26. Frasnelli J, Ungermann M, Hummel T. Ortho‐ and retronasal presentation of olfactory stimuli modulates odor percepts. Chemosens Percept. 2008;1:9‐15. [Google Scholar]
- 27. Kjaergaard T, Cvancarova M, Steinsvåg SK. Does nasal obstruction mean that the nose is obstructed? Laryngoscope. 2008;118:1476‐1481. [DOI] [PubMed] [Google Scholar]
- 28. Ottaviano G, Pendolino AL, Nardello E, et al. Peak nasal inspiratory flow measurement and visual analogue scale in a large adult population. Clin Otolaryngol. 2019;44:541‐548. [DOI] [PubMed] [Google Scholar]
- 29. Widiarni D, Paramyta WW, Wardani RS, Bachtiar A. Comparison of nasal obstruction symptom evaluation, peak nasal inspiratory flowmeter, and rhinomanometry in patients with nasal deformities. J Phys Conf Ser. 2018;1073:022024 https://iopscience.iop.org/article/10.1088/1742-6596/1073/2/022024/pdf. [Google Scholar]
- 30. van Egmond MMHT, Rovers MM, Hannink G, Hendriks CTM, van Heerbeek N. Septoplasty with or without concurrent turbinate surgery versus non‐surgical management for nasal obstruction in adults with a deviated septum: a pragmatic, randomised controlled trial. Lancet. 2019;394:314‐321. [DOI] [PubMed] [Google Scholar]


