Fig. 1.
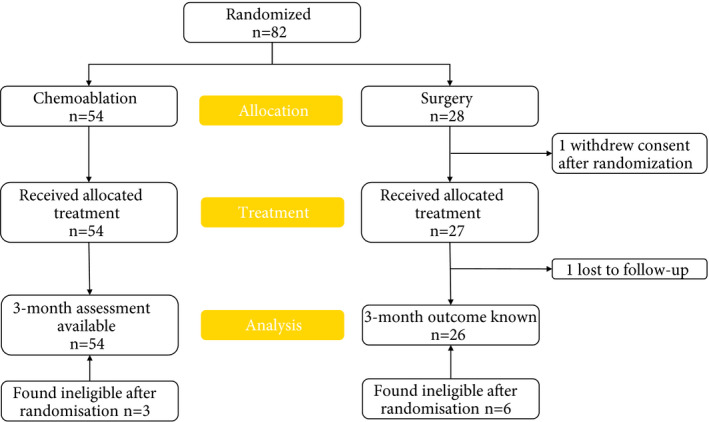
CONSORT diagram. Eighty patients were included in the primary and efficacy endpoints’ analysis: two patients without a 3‐month assessment in the surgical management group were excluded (one withdrew from trial treatment after randomization, one was lost to follow‐up before 3 months). All patients for whom there were completed post‐treatment and/or 3‐month adverse event forms were included in the safety analyses (N = 81). Nine patients (three surgical management, six chemoablation) were found ineligible after randomization but were included in all analyses in accordance with the CALIBER Statistical Analysis Plan.
