Figure 9.
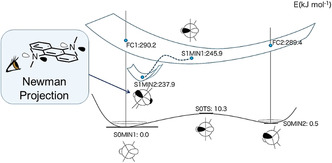
Schematic illustration of the PES of 9,10‐bis(N,N‐dimethylamino)anthracene obtained from DFT/TD‐DFT calculations at the B3LYP/6‐31+G(d) level of theory. The ground state adopts two stable conformations (S0MIN) that are interconverted through the inversion of its NR3 tetrahedra. After photoexcitation, each Franck–Condon (FC) state relaxes through planarization of one NR3, leading to the conformation shown as S1MIN1. This “umbrella motion” occurs also at the other NR3 tetrahedron under concomitant tilting of these planar trigonal planes, which gives another stable conformation (S1MIN2). These structural relaxations decrease the S0–S1 transition energy to the extent comparable with the experimental Stokes shifts. Any MECI was not sampled in this calculation and is hence not shown here.
