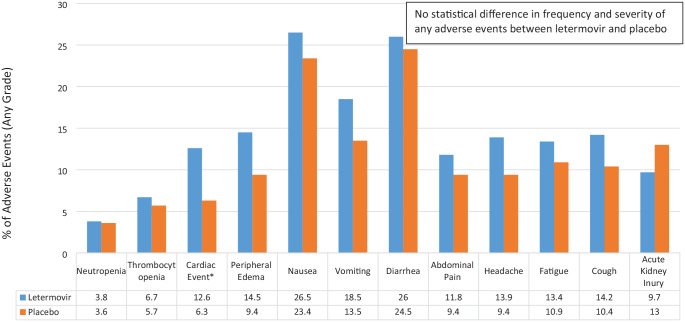
Figure 1.
Select LTV versus placebo adverse events (any grade) reported in phase III trial.16
*Cardiac event included (LTV versus placebo): atrial fibrillation (3.5% versus 1%), atrial flutter (1.1% versus 0%), cardiac failure (1.3% versus 0%), sinus tachycardia (1.1% versus 1.6%), and tachycardia (4% versus 2.1%). Further analysis did not show a relationship between letermovir and atrial arrhythmias.
LTV, letermovir.