Abstract
Transarterial chemoembolization using doxorubicin (TACE-DOX) is an effective therapy for advanced hepatocellular carcinoma (HCC). However, there are limited options for patients with TACE refractoriness. We compared the effectiveness between sorafenib and transarterial chemolipiodolization using epirubicin and cisplatin combined with systemic infusion of 5-fluorouracil (5-FU; TACL-ECF) in patients with previous TACE-DOX refractoriness. We retrospectively analyzed 742 consecutively enrolled cohort patients who received TACE-DOX as the first-line therapy for HCC. Among the 94 patients who failed with TACE-DOX, 49 patients were treated with TACL-ECF and 45 patients were treated with sorafenib as a rescue therapy. The TACL-ECF regimen comprised transarterial infusion of epirubicin and cisplatin combined with systemic infusion of 5-FU. Of the 94 patients, 22 and 72 patients were in Barcelona Clinic Liver Cancer stages B and C, respectively; 66% patients were classified as having Child-Pugh class A (CPC A). Overall survival (OS) after rescue therapy did not differ between the sorafenib and TACL-ECF groups (4.1 months vs 6.4 months, P = .355). Progression-free survival (PFS) did not differ between the sorafenib and TACL-ECF groups (2.8 months vs 3.5 months, P = .629). Adverse events of CTC grade 3/4 occurred more frequently in the sorafenib group than in the TACL-ECF group (P = .024). The present study showed that the OS and PFS did not differ between patients given rescue TACL-ECF therapy and those given sorafenib therapy. The TACL-ECF treatment was better tolerated than sorafenib. The TACL-ECF might be considered as an alternative therapy for the patients with TACE-DOX refractoriness, especially CPC B and sorafenib-intolerant patients.
Keywords: hepatocellular carcinoma, 5-fluorouracil, cisplatin, epirubicin, sorafenib, transarterial chemoembolization refractoriness
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cancer worldwide and the most common primary liver cancer.1 Although there are a variety of therapies for the treatment of HCC, curative treatments such as surgical resection, liver transplantation, and radiofrequency ablation can be used in only one-third of patients with HCC.2 In patients who are not candidates for curative treatment of HCC, transarterial chemoembolization (TACE) is one of the most commonly used treatment modalities. Patients with Barcelona Clinic Liver Cancer (BCLC) stage B HCC who have taken TACE have a median overall survival (OS) of 26 months.3-5 However, the prognosis in advanced HCC remains poor, especially for those with TACE refractoriness.
For patients who experience TACE refractoriness, sorafenib is recommended for rescue therapy in several guidelines.2,6 In a comparison of sorafenib with steady TACE in patients with intermediate-stage HCC that is refractory to TACE, sorafenib treatment was associated with a longer OS and time to progression compared with steady TACE.7 In patients with locally advanced HCC or refractoriness to TACE, transarterial radioembolization (TARE) is also available.8 Some studies comparing the efficacy of TARE and sorafenib have shown that TARE is a safer and more effective treatment than sorafenib.9-11 However, there is still controversy about the efficacy of TARE, and there are also patients who are not eligible for TARE.12,13
Transarterial chemoembolization induces ischemic tumor necrosis by obstructing the hepatic artery and exerts an anticancer effect via chemotherapeutic agents such as doxorubicin (DOX), epirubicin, or cisplatin mixed with lipiodol. Doxorubicin is the most commonly used chemotherapeutic agent in TACE for HCC.4,5 Ellis et al14 reported that epirubicin, cisplatin, and continuous 5-flurouracil (5-FU) were novel therapy for hepatobiliary tumors and were well tolerated. And in later reported paper of TACE using epirubicin, cisplatin, and 5-FU produced better survival benefits than TACE with DOX.15 Studies of hepatic arterial infusion chemotherapy using cisplatin and interferon for HCC refractory to TACE have produced controversial results.16,17 Tumor progression after TACE refractoriness is expressed as intrahepatic or extrahepatic tumor progression. Although sorafenib has systemic anticancer effects, TACE is mainly a treatment for intrahepatic tumors. We tried to obtain both an intrahepatic embolic effect and systemic anticancer effect using multiple anticancer drugs, such as epirubicin, cisplatin, and 5-FU.
We assessed the effectiveness and safety of a protocol using transarterial chemolipiodolization (TACL) combined with epirubicin and cisplatin with systemic infusion of 5-FU (TACL-ECF) compared with sorafenib as the rescue therapy in patients with HCC refractory to TACE-DOX.
Materials and Methods
Study Population
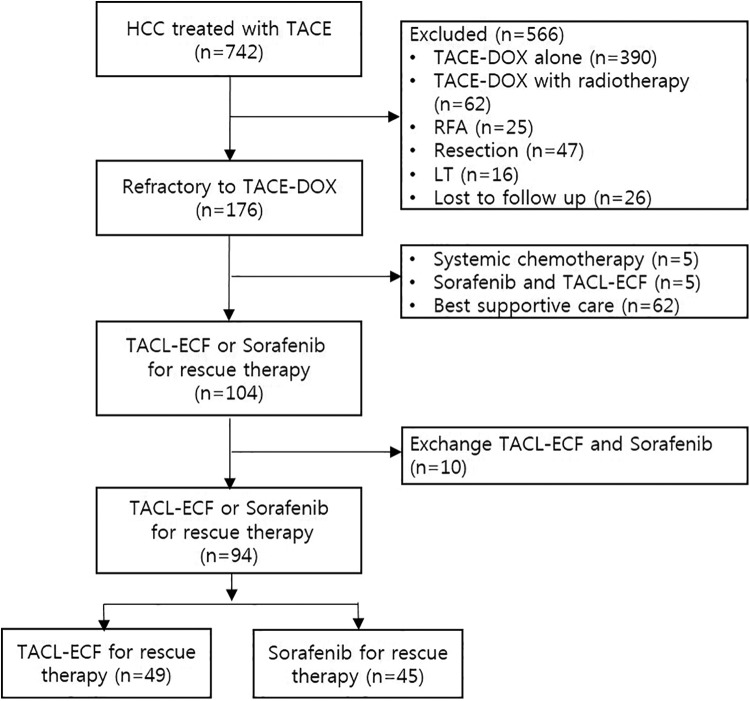
This was a retrospective observational study of 742 patients within a prospectively collected cohort who underwent TACE-DOX as the first-line therapy for HCC at the Catholic University of Korea Incheon St. Mary’s Hospital between January 2007 and December 2017 (Figure 1). Primary HCC was diagnosed in 1238 patients in our institution. The diagnosis of HCC was made on the basis of the American Association for the Study of Liver Diseases practice guidelines.18 Patients who met the following criteria were entered into the study: age ≥18 years, Child-Pugh class (CPC) A or B status, TACE as the first-line therapy, and TACL-ECF or sorafenib as a rescue therapy. TACE refractoriness was defined as an ineffective response after 2 or more consecutive TACE procedures as shown by the response evaluation to computed tomography (CT) or magnetic resonance imaging (MRI) 1 to 3 months after treatment according to the Japan Society of Hepatology Criteria 2014 Update.19 Patients were excluded for the following reasons: the presence of another primary tumor; CPC C status; BCLC stage D; combined therapy with radiotherapy (n = 62); other sequential therapy for patients with response after TACE-DOX including resection (n = 47), radiofrequency ablation (n = 25), or liver transplantation (n = 16); other sequential therapy for patients with refractoriness to TACE-DOX including systemic chemotherapy (n = 5), combination of TACL-ECF and sorafenib (n = 5), or best supportive care (n = 62); or HCC treated with sorafenib in the TACL-ECF group and TACL-ECF in the sorafenib group (n = 10). The final analysis included 94 patients with HCC treated with TACL-ECF (n = 49) or sorafenib (n = 45) as a rescue therapy after TACE-DOX refractoriness (Figure 1).
Figure 1.
Flow diagram for the patient selection process.
This retrospective cohort study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the local ethics committee and the institutional review board of the Catholic University of Korea (OC17RISI0004). The need of informed consent was waived by the local ethic committee and the institutional review board of the Catholic University of Korea because clinical data were analyzed retrospectively and anonymously in this study.
Treatment Protocol
In the TACL-ECF group, the femoral artery was catheterized under fluoroscopy. A hepatic arteriogram was performed to detect the feeding arteries of the HCC and for distal superselective catheterization. Patients in this treatment group received a transarterial infusion of epirubicin (50 mg/m2) and cisplatin (60 mg/m2) in a mixture of 5 to 10 mL of iodized oil (Lipiodol Ultra Fluid; Guerbet) without Gelfoam embolization. After the transarterial procedure, the patient received an additional systemic infusion of 5-FU (200 mg/m2) for 12 hours. Unless there was a contraindication, the combination treatment sessions were performed every 2 months. The dose or treatment interval was modified whenever any treatment-related toxicity was encountered. The treatment response was assessed 1 to 2 months after each TACL-ECF treatment using dynamic CT or MRI.
In the sorafenib group, the initial dose of sorafenib was 400 mg twice daily. Dose reductions were based on the presence of toxicity and a 2-step dose reduction was allowed (from 400 mg once daily to 400 mg once every other day). Administration of sorafenib continued until the patient experienced severe adverse events or disease progression was apparent. Patients who received at least 1 dose of sorafenib were included in the sorafenib group. The treatment response was assessed every 2 months using dynamic CT or MRI.
Assessment of the Treatment Response and Toxicity
Dynamic CT or MRI was performed 1 to 2 months after each TACL-ECF treatment and every 2 months after administration of sorafenib. The modified Response Evaluation Criteria in Solid Tumors guideline20 was used to assess the treatment response as follows: complete response, defined as disappearance of any intratumoral arterial enhancement in all target lesions; partial response (PR), defined as at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions; progressive disease (PD), defined as an increase of at least 20% in the sum of the diameters of viable target lesions; or stable disease (SD), any case that did not qualify as either PR or PD. Adverse drug reactions were recorded during the treatment and 1 week after each treatment and were defined according to the Common Terminology Criteria (CTC) of Adverse Events, version 4.0.
Statistical Analyses
Categorical variables are described as frequencies and percentages. Continuous variables are presented as mean ± SD or median with interquartile range for parametric and nonparametric variables, respectively. The baseline characteristics were analyzed using the χ2 test for categorical variables and Student t test for continuous variables. Kaplan-Meier curves and log-rank tests were used to estimate survival and tumor progression. Cox proportional hazards model was used to identify the risk factors for survival and tumor progression. The variables included in the multivariate analysis were selected on the basis of statistical significance in the univariate analysis (P < .05). A P value <.05 in a 2-tailed test was regarded as significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 16.0 for Windows (SPSS Statistics).
Results
Baseline Characteristics of the Patients
The baseline characteristics of the study population are presented in Table 1. The study included 94 patients with HCC treated with TACL-ECF (n = 49) or sorafenib (n = 45) as a rescue therapy after TACE-DOX refractoriness. The median follow-up period was 5.8 months (1.0-35.7 months). The median duration of sorafenib administration was 2.8 months (1.0-17.8 months), and the median number of TACL-ECF treatments was 2 (1-10). Of the 94 patients, 22 and 72 were in BCLC stages B and C, respectively, and the BCLC stage did not differ significantly between the TACL-ECF group and sorafenib group (P = .813). The CPC stage at the baseline did not differ between groups (P = .829); 66% of patients were classified as CPC A. The tumor characteristics including maximum tumor size, portal vein invasion, extrahepatic metastasis, and α-fetoprotein (AFP) level did not differ significantly between the TACL-ECF and sorafenib groups.
Table 1.
Baseline Characteristics of the Study Population.a
| All patients, n = 94 | TACL-ECF, n = 49 | Sorafenib group, n = 45 | P value | |
|---|---|---|---|---|
| Males, n (%) | 70 (74.4) | 37 (75.5) | 33 (73.3) | .818 |
| Age, years (median, range) | 59 (41-80) | 58 (42-76) | 61 (41-80) | .243 |
| Underlying disease, n (%) | .064 | |||
| Chronic hepatitis B | 70 (74.5) | 39 (79.6) | 31 (68.9) | |
| Chronic hepatitis C | 14 (14.9) | 9 (18.4) | 5 (11.1) | |
| Alcoholic liver disease | 4 (4.2) | 1 (2.0) | 3 (6.7) | |
| Others | 6 (6.4) | 0 (0.0) | 6 (13.3) | |
| Child-Pugh class, n (%) | .829 | |||
| A | 62 (66.0) | 33 (67.3) | 29 (64.4) | |
| B | 32 (34.0) | 16 (32.7) | 16 (35.6) | |
| BCLC stage, n (%) | .813 | |||
| B | 22 (23.4) | 12 (24.5) | 10 (22.2) | |
| C | 72 (76.6) | 37 (75.5) | 35 (77.8) | |
| Maximum tumor diameter (cm), n (%) | .153 | |||
| >5 | 51 (54.3) | 23 (46.9) | 28 (62.2) | |
| ≤5 | 43 (45.7) | 26 (53.1) | 17 (37.8) | |
| PV invasion, n (%) | .294 | |||
| Yes | 56 (59.6) | 32 (65.3) | 24 (53.3) | |
| No | 38 (40.4) | 17 (34.7) | 21 (46.7) | |
| Extrahepatic metastases, n (%) | .214 | |||
| Yes | 43 (45.7) | 19 (38.8) | 24 (53.3) | |
| No | 51 (54.3) | 30 (61.2) | 21 (46.7) | |
| AFP (ng/dL), n (%) | .837 | |||
| >200 | 53 (56) | 27 (55.1) | 26 (57.8) | |
| ≤200 | 41 (43.6) | 22 (44.9) | 19 (42.2) | |
| ALT (U/L), (mean ± SD) | 38.61 ± 23.52 | 40.22 ± 25.39 | 36.84 ± 21.45 | .489 |
| Platelets (×1000/mm3), mean ± SD | 145 ± 101 | 126 ± 73 | 165 ± 123 | .059 |
| Bilirubin (mg/dL), (mean ± SD) | 1.20 ± 0.69 | 1.14 ± 0.63 | 1.26 ± 0.75 | .364 |
| Albumin (g/dL), (mean ± SD) | 3.38 ± 3.40 | 3.27 ± 0.67 | 3.50 ± 0.47 | .056 |
| PT INR, (mean ± SD) | 1.18 ± 0.14 | 1.19 ± 0.15 | 1.16 ± 0.12 | .351 |
| Creatinine (mg/dL), (mean ± SD) | 0.74 ± 0.19 | 0.75 ± 0.19 | 0.72 ± 0.20 | .451 |
Abbreviations: AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; PT INR, prothrombin time international normalized ratio.
a The χ2 test for categorical variables and Student t test for continuous variables. Data are presented as the mean ± SD, median (range in parentheses) or number of patients (percentages in parentheses).
Analysis of the Effectiveness of TACL-ECF and Sorafenib
Follow-up imaging as the assessment of tumor response at 2 months after treatment was available for 42 (85.7%) of 49 patients in the TACL-ECF group and 36 (80.0%) of 45 patients in the sorafenib group. In the TACL-ECF group, PR was achieved in 1 (2.0%) patient, SD in 25 (51.0%) patients, and PD in 16 (32.7%) patients. In the sorafenib group, PR was achieved in 2 (4.4%) patients, SD in 18 (40.0%) patients, and PD in 16 (35.6%) patients. The tumor response did not differ between the 2 groups (P = .670).
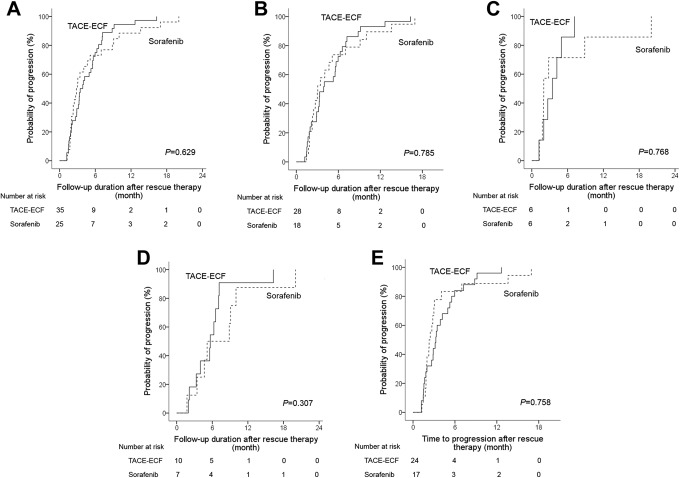
The median time to progression did not differ between the TACL-ECF group (3.5 months; 95% CI: 2.4-4.5) and sorafenib group (2.8 months; 95% CI: 2.1-3.6; P = .629; Figure 2). Subgroup analysis showed that the median time to progression did not differ significantly between the TACL-ECF group and sorafenib group within the CPC A (3.9 months vs 3.1 months, P = .785), CPC B (3.5 months vs 2.0 months, P = .768), BCLC B (5.7 months vs 5.1 months, P = .307), and BCLC C (3.3 months vs 2.3 months, P = .758) groups (Figure 2B-E). Multivariate analysis of the risk estimation showed that being male (P = .047; hazard ratio [HR]: 1.892; 95% CI: 1.008-3.553) or having BCLC C HCC (P = .022; HR: 1.925; 95% CI: 1.098-3.373) was associated with a more rapid progression of HCC in both groups (Table 2).
Figure 2.
Kaplan-Meier curves of the time to progression between TACL-ECF and sorafenib treatment groups in total patients (A), CPC A (B), CPC B (C), BCLC B (D), and BCLC C (E). BCLC indicates Barcelona Clinic Liver Cancer; CPC, Child-Pugh class; ECF, epirubicin and cisplatin combined with systemic infusion of 5-fluorouracil; TACL, transarterial chemolipiodolization.
Table 2.
Factors Associated With Progression.a
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender (male vs female) | 1.945 (1.046-3.616) | .036 | 1.892 (1.008-3.553) | .047 |
| Age, years (>60 vs ≤60) | 0.894 (0.523-1.527) | .681 | ||
| Rescue therapy (TACL-ECF vs Sorafenib) | 0.879 (0.519-1.488) | .630 | ||
| Child-Pugh class (B/A) | 1.084 (0.584-2.011) | .798 | ||
| BCLC stage (C/B) | 1.957 (1.121-3.416) | .018 | 1.925 (1.098-3.373) | .022 |
| Serum AFP, ng/mL (>200 vs ≤200) | 1.167 (0.697-1.954) | .557 | ||
Abbreviations: AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio.
a Cox proportional hazards model.
Survival Analysis After Rescue Therapy
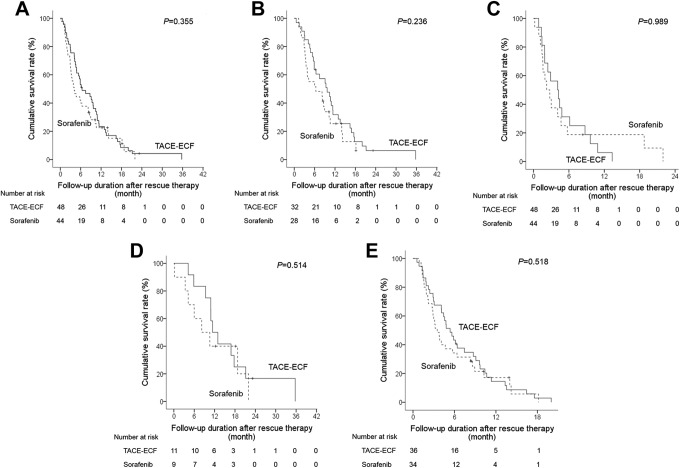
During the follow-up period, 87 of 94 patients died because of tumor progression. The median survival time was 6.4 months (95% CI: 2.9-9.9) in the TACL-ECF group and 4.1 months (95% CI: 2.6-5.6) in the sorafenib group (P = .355; Figure 3A). Overall survival was related to being male (P = .017; HR: 1.812; 95% CI: 1.115-2.947), having CPC B HCC (P = .002; HR: 2.037; 95% CI: 1.302-3.187), having BCLC stage C HCC (P < .0001; HR: 3.023; 95% CI: 1.709-5.345), and having a serum AFP level >200 ng/mL (P = .022; HR: 1.661; 95% CI: 1.076-2.563). In the multivariate analysis, CPC B HCC (P = .004; HR: 1.976; 95% CI: 1.240-3.150) and BCLC stage C HCC (P < .0001; HR: 2.965; 95% CI: 1.638-5.364) were negative predictors of OS (Table 3).
Figure 3.
Kaplan-Meier curves of the overall survival between TACL-ECF and sorafenib treatment groups in total patients (A), CPC A (B), CPC B (C), BCLC B (D), and BCLC C (E). BCLC indicates Barcelona Clinic Liver Cancer; CPC, Child-Pugh class; ECF, epirubicin and cisplatin combined with systemic infusion of 5-fluorouracil; TACL, transarterial chemolipiodolization.
Table 3.
Factors Associated With Overall Survival.a
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender (male vs female) | 1.812 (1.115-2.947) | .017 | 1.521 (0.923-2.506) | .100 |
| Age, years (>60 vs ≤60) | 1.120 (0.729-1.721) | .605 | ||
| Rescue therapy (TACL-ECF vs Sorafenib) | 0.817 (0.532-1.255) | .357 | ||
| Child-Pugh class (B/A) | 2.037 (1.302-3.187) | .002 | 1.976 (1.240-3.150) | .004 |
| BCLC stage (C/B) | 3.023 (1.709-5.345) | <.0001 | 2.965 (1.638-5.364) | <.0001 |
| Serum AFP, ng/mL (>200 vs ≤200) | 1.661 (1.076-2.563) | .022 | 1.157 (0.734-1.823) | .531 |
Abbreviations: AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio.
a Cox proportional hazards model.
In the subgroup analysis, overall survival did not differ between the TACL-ECF group and sorafenib group within the CPC A (9.7 months vs 6.4 months, P = .236), CPC B (4.1 months vs 2.2 months, P = .989), BCLC stage B (11.4 months vs 8.2 months, P = .514), and BCLC stage C (5.4 months vs 3.5 months, P = .518) subgroups (Figure 3B-E).
Analysis of Treatment-Related Safety
Treatment-related adverse events of CTC ≥3 occurred in 6 (12.2%) of 49 patients in the TACL-ECF group and 15 (33.3%) of 45 patients in the sorafenib group (P = .024; Table 4). In patients with Child-Pugh class A, adverse events of CTC ≥3 more occurred in sorafenib group than in TACL-ECF group (27.6% vs 6.1%, P = .036). Discontinuation of treatment because of treatment-related adverse events occurred in significantly more patients in the sorafenib group (14/patients, 31.1%) than in the TACL-ECF group (6/49 patients, 12.2%; P = .042).
Table 4.
Adverse Events of Grade ≥3 During the Observation Period.
| All, n = 94 | Child-Pugh class A, n = 62 | Child-Pugh class B, n = 32 | ||||
|---|---|---|---|---|---|---|
| TACL-ECF, n = 49 | Sorafenib group, n = 45 | TACL-ECF, n = 33 | Sorafenib group, n = 29 | TACL-ECF, n = 16 | Sorafenib group, n = 16 | |
| Nausea/vomiting | 1 (2.0%) | 1 (2.2%) | 0 (0%) | 0 (0%) | 1 (6.3%) | 1 (6.3%) |
| Fatigue | 1 (2.0%) | 1 (2.2%) | 0 (0%) | 1 (3.4%) | 1 (6.3%) | 0 (0%) |
| Hand-foot syndrome | 0 (0%) | 4 (8.9%) | 0 (0%) | 3 (10.3%) | 0 (0%) | 1 (6.3%) |
| Elevated aspartate aminotransferase | 3 (6.1%) | 6 (13.3%) | 1 (3.0%) | 2 (6.9%) | 2 (12.5%) | 4 (25.0%) |
| Bone marrow suppression | 1 (2.0%) | 0 (0%) | 1 (3.0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hepatic encephalopathy | 0 (0%) | 1 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6.3%) |
| Variceal bleed | 0 (0%) | 2 (4.4%) | 0 (0%) | 1 (3.4%) | 0 (0%) | 1 (6.3%) |
| Pulmonary thromboembolism | 0 (0%) | 1 (2.2%) | 0 (0%) | 1 (3.4%) | 0 (0%) | 0 (0%) |
Discussion
Transarterial chemoembolization is a treatment of choice for patients with intermediate-stage HCC. In a randomized controlled study, TACE showed survival benefits in patients with unresectable HCC not suitable for curative treatment compared with the best supportive treatment. However, only about 60% of patients respond to TACE as the first-line therapy of HCC, and about 40% of patients experience tumor progression after TACE.5 The effects of sorafenib for rescue therapy in patients with HCC refractory to TACE have more favorable survival outcomes than those who continue TACE.7 The Japan Society of Hepatology and the European Association for the Study of the Liver suggest the use of sorafenib treatment as the rescue therapy after TACE refractoriness.2,19 The current guidelines for management of HCC systemic therapies are recommended for advanced HCC or HCC with refractoriness to TACE. Sorafenib and lenvatinib have been shown to be effective in first line, and regorafenib, cabozantinib, nivolumab, ramucirumab, and pembrolizumab have been approved in the second line.8,18,21 However, all these systemic therapies are allowed in patients with CPC A. Therefore, we compared the effectiveness and safety between sorafenib and TACL-ECF in patients with HCC who were refractory to TACE-DOX.
In the present study, we found a consistently comparable effectiveness of TACL-ECF versus sorafenib for patients with previous TACE-DOX refractoriness. In these patients with TACE refractoriness, the median time to progression of the TACL-ECF treatment group was not inferior to that of the sorafenib group (3.5 months vs 2.8 months, P = .629). In addition, the median OS times did not differ between the groups: 6.4 and 4.1 months, respectively, for the group of patients who received rescue therapy after TACE-DOX refractoriness for TACL-ECF and the sorafenib group (P = .355). The survival outcomes of the TACL-ECF treatment group were similar to those of the sorafenib treatment group in terms of time to progression and OS. The OS after hepatic arterial infusion chemotherapy was also not inferior to that after sorafenib for the treatment of unresectable advanced HCC in other studies.22-25
In our study, we investigated the use of an alternative option in patients with CPC B HCC after previous TACE refractoriness. We found a shorter survival time than that reported in previous studies. The OS in our study was 4.1 to 6.4 months, whereas other practice-based studies have reported survival of 7.4 to 25.4 months in patients, given sorafenib as rescue therapy after TACE refractoriness.7,16,17,26,27 Discrepancy may reflect differences in the distribution of patients between our study and the previous studies. In the present study, we included patients with CPC B (34.0%) and BCLC stage C (76.6%) HCC, whereas previous studies had excluded patients with CPC B of BCLC stage C HCC. In general, patients with advanced HCC have worse liver function. However, sorafenib has been approved for use in patients with CPC A with unresectable HCC. Therefore, there are limited options in clinical practice for patients with advanced HCC and poor liver function such as those with CPC B HCC.
In our study, adverse events of CTC grade 3/4 occurred more frequently in the sorafenib group than in the TACL-ECF group (33.3% vs 12.2%, P = .024). Prospective randomized studies of the use of sorafenib for HCC have reported that 29.9 to 43.9% Asian patients experienced adverse events of CTC grade 3 or 4.28,29 The TACL-ECF treatment was better tolerated than sorafenib by patients with HCC who were refractory to TACE-DOX. The recently approved drugs for advanced HCC, such as sorafenib, regorafenib, and lenvatinib, can be used to treat patients with CPC A. The recent development of antiviral drugs has meant that even patients with cirrhosis with advanced HCC can maintain liver function up to CPC B disease. In our study, we found similar effectiveness and fewer safety issues in the TACL-ECF group compared with the sorafenib group.
Our study has several limitations. First, this was a retrospective observational study and it may have included some bias in the comparison of the TACL-ECF and sorafenib groups. However, there were no differences in baseline characteristics relating to the patients and tumors between the 2 treatment groups. To minimize the potential for bias, COX regression was used to identify the factors related to survival outcomes. Second, because the order of sequential therapy may affect the survival outcomes, we excluded patients who had received sorafenib before TACL-ECF or TACL-ECF before sorafenib sequential therapy. Further work is needed to determine the responses to anticancer therapy and whether the responses differ according to the order of sequential therapy in clinical practice.
In conclusion, the time to progression and OS of the TACL-ECF group was not inferior to that of the sorafenib group. The TACL-ECF might be considered as an alternative therapy for patients with TACE-DOX refractoriness, especially patients with CPC B who are not the candidates for sorafenib therapy. The TACE-DOX treatment was better tolerated than sorafenib in terms of adverse events. The TACL-ECF may be an alternative treatment option for patients who are refractory to TACE-DOX but who cannot tolerate sorafenib.
Footnotes
Authors’ Note: This retrospective cohort study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the local ethics committee and the institutional review board of the Catholic University of Korea (OC17RISI0004). The need of informed consent was waived by the local ethic committee and the institutional review board of the Catholic University of Korea because clinical data were analyzed retrospectively and anonymously in this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jung Hyun Kwon  https://orcid.org/0000-0002-5484-5864
https://orcid.org/0000-0002-5484-5864
References
- 1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi:10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 2. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi:10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 3. Ikai I, Arii S, Ichida T, et al. Report of the 16th follow-up survey of primary liver cancer. Hepatol Res. 2005;32(3):163–172. doi:10.1016/j.hepres.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 4. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatol (Baltimore, Md) 2011;53(3):1020–1022. doi:10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet (Lond, Engl). 2002;359(9319):1734–1739. doi:10.1016/s0140-6736(02)08649-x [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Digest Dis (Basel, Switzerland). 2011;29(3):339–364. doi:10.1159/000327577 [DOI] [PubMed] [Google Scholar]
- 7. Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014;87(6): 330–341. doi:10.1159/000365993 [DOI] [PubMed] [Google Scholar]
- 8. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 9. Kim PH, Choi SH, Kim JH, Park SH. Comparison of radioembolization and sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis of safety and efficacy. Kor J Radiol. 2019;20(3):385–398. doi:10.3348/kjr.2018.0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rognoni C, Ciani O, Sommariva S, et al. Trans-arterial radioembolization for intermediate-advanced hepatocellular carcinoma: a budget impact analysis. BMC Cancer. 2018;18:715 doi:10.1186/s12885-018-4636-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhan C, Ruohoniemi D, Shanbhogue KP, et al. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(1):25–34. doi:10.1016/j.jvir.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 12. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636. doi:10.1016/s1470-2045(17)30683-6 [DOI] [PubMed] [Google Scholar]
- 13. Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–1921. doi:10.1200/jco.2017.76.0892 [DOI] [PubMed] [Google Scholar]
- 14. Ellis PA, Norman A, Hill A, et al. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer (Oxford, England: 1990). 1995; 31a(10):1594–1598. doi:10.1016/0959-8049(95)00323-b [DOI] [PubMed] [Google Scholar]
- 15. Lee SW, Lee HL, Han NI, et al. Transarterial infusion of epirubicin and cisplatin combined with systemic infusion of 5-fluorouracil versus transarterial chemoembolization using doxorubicin for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a retrospective analysis. Ther Adv Med Oncol. 2017;9(10):615–626. doi: 10.1177/1758834017728018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondo M, Morimoto M, Ishii T, et al. Hepatic arterial infusion chemotherapy with cisplatin and sorafenib in hepatocellular carcinoma patients unresponsive to transarterial chemoembolization: a propensity score-based weighting. J Dig Dis. 2015;16(3):143–151. doi:10.1111/1751-2980.12221 [DOI] [PubMed] [Google Scholar]
- 17. Hatooka M, Kawaoka T, Aikata H, et al. Comparison of outcome of hepatic arterial infusion chemotherapy and sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Anticancer Res. 2016;36(7):3523–3529 [PubMed] [Google Scholar]
- 18. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2018;68(2):723–750. doi:10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 19. Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3(3-4):458–468. doi:10.1159/000343875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 21. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv238–iv255. doi:10.1093/annonc/mdy308 [DOI] [PubMed] [Google Scholar]
- 22. Hiramine Y, Uto H, Imamura Y, et al. Sorafenib and hepatic arterial infusion chemotherapy for unresectable advanced hepatocellular carcinoma: a comparative study. Exp Ther Med. 2011;2(3):433–441. doi:10.3892/etm.2011.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeong SW, Jang JY, Lee JE, et al. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia-Pac J Clin Oncol. 2012;8(2):164–171. doi:10.1111/j.1743-7563.2012.01543.x [DOI] [PubMed] [Google Scholar]
- 24. Kim HY, Park JW, Nam BH, et al. Survival of patients with advanced hepatocellular carcinoma: sorafenib versus other treatments. J Gastroenterol Hepatol. 2011;26(11):1612–1618. doi:10.1111/j.1440-1746.2011.06751.x [DOI] [PubMed] [Google Scholar]
- 25. Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50(4):445–454. doi:10.1007/s00535-014-0978-3 [DOI] [PubMed] [Google Scholar]
- 26. Lee S, Kang JH, Kim DY, et al. Prognostic factors of sorafenib therapy in hepatocellular carcinoma patients with failure of transarterial chemoembolization. Hepatol Int. 2017;11(3):292–299. doi:10.1007/s12072-017-9792-3 [DOI] [PubMed] [Google Scholar]
- 27. Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262. doi:10.1159/000367743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim DY, Kim HJ, Han KH, et al. Real-life experience of sorafenib treatment for hepatocellular carcinoma in Korea: from GIDEON data. Cancer Res Treat. 2016;48(4):1243–1252. doi:10.4143/crt.2015.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kudo M, Ikeda M, Takayama T, et al. Safety and efficacy of sorafenib in Japanese patients with hepatocellular carcinoma in clinical practice: a subgroup analysis of GIDEON. J Gastroenterol. 2016;51(12):1150–1160. doi:10.1007/s00535-016-1204-2 [DOI] [PMC free article] [PubMed] [Google Scholar]