Abstract
Background
Glioblastoma (GBM) is associated with poor prognosis, large morbidity burden, and limited treatment options. This analysis evaluated real-world treatment patterns, overall survival, resource use, and costs among Medicare patients with GBM.
Methods
This retrospective observational study evaluated Medicare patients age 66 years or older with newly diagnosed GBM using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data from 2007 through 2013. Patients were followed from diagnosis to death or end of follow-up. An algorithm defined treatment patterns as lines of therapy (LOTs). The Kaplan-Meier method was used to estimate overall survival for the full sample as well as by LOT, surgical resection, Charlson Comorbidity Index (CCI), tumor size, and age. Resource use and costs during the follow-up period were reported in terms of total and per-patient-per-month (PPPM) estimates.
Results
A total of 4308 patients with GBM were identified (median age, 74 years; CCI of 0, 52%). The most commonly used first LOT was temozolomide (82%), whereas chemotherapy + bevacizumab was most prevalent for second-line (42%) and third-line (58%) therapy. The median overall survival was 5.9 months for resected patients and 3 months for unresected patients, with considerable heterogeneity depending on patient characteristics. A great proportion of patients had claims for an ICU admission (86.2%), skilled nursing facility (76.9%), and home health (56.0%) in the postdiagnosis period. The cumulative mean cost was $95 377 per patient and $18 053 PPPM, mostly attributed to hospitalizations.
Conclusions
Limited treatment options, poor survival, and economic burden emphasize the need for novel interventions to improve care for Medicare patients with GBM.
Keywords: costs, glioblastoma, line of therapy, resource use, survival
Glioblastoma (GBM), formerly glioblastoma multiforme, is the most common adult malignant primary brain tumor, representing 54% of all gliomas and 45% of malignant primary brain and CNS tumors.1 About half of patients who are newly diagnosed with GBM are older than 64 years. The annual incidence of GBM is 3.19 cases per 100 000 people in the United States and is greatest in patients age 75 to 84 years (15.03 cases per 100 000 people).2,3
Without intervention, patients with GBM die shortly after diagnosis.4 The median overall survival (mOS) for patients with GBM dramatically increases with standard of care first-line (1L) treatment consisting of maximal safe resection followed by radiotherapy (RT) with concurrent temozolomide (TMZ), followed by adjuvant TMZ.5 Based on their landmark clinical trial, Stupp et al reported a mOS of 14.6 months with RT plus TMZ and 12.1 months with RT alone.5 However, more than 50% of patients with GBM experience disease recurrence within 7 months of initiating 1L treatment.6 Prognosis is even poorer for recurrent GBM, with a 6-month and 12-month OS rate of 60% to 70%6–10 and 20% to 30%, respectively.6,9,11–13 No standard of care has been established in the second-line (2L) setting.
Treatment of GBM in older patients is more challenging because of their poorer prognosis and higher comorbidities. In addition, older patients with GBM may have increased risk of brain toxicity due to RT14; however, there have been some studies suggesting reducing the dose and duration of RT, which may reduce toxicity without a significant impact on survival.15,16 Because the trial by Stupp and colleagues excluded patients 70 years and older, no clear standard of care exists for older patients with GBM. A more recent clinical trial by Perry et al randomly assigned elderly patients with GBM to receive short courses of RT alone or RT with concomitant and adjuvant TMZ. They report a mOS of 9.3 months with RT plus TMZ and 7.6 months with RT alone.17
Although the economic burden of GBM has been previously studied,18–23 the economic trajectories of patients with GBM receiving sequential lines of therapy (LOTs) during their disease have not been previously evaluated. This is relevant because the majority of patients will invariably require several (sequential) LOTs because of disease progression. Furthermore, it will be important to understand the economic impact of the traditional systemic therapies in the context of an evolving treatment landscape, including the evaluation of vaccination therapy, checkpoint inhibitors, T-cell therapies, combinations of immunotherapies, and tumor-treating field therapy.24,25 This analysis was conducted to assess the real-world treatment patterns, overall survival, health care resource use (HCRU), and direct medical costs in US Medicare patients newly diagnosed with GBM.
Methods
Data Source
This study used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database between 2007 and 2013. The SEER-Medicare database links information from the US National Cancer Institute’s 18 SEER cancer registries and Medicare claims data from the Centers for Medicare and Medicaid Services. The SEER program registry collects cancer incidence and mortality rates from 18 tumor registries across the United States covering 28% of the population.26,27 This registry contains data on patient demographics, primary tumor site, tumor morphology, and follow-up for vital status. Medicare claims provide information on health care services that are provided to and covered for Medicare beneficiaries from the time of Medicare eligibility until death.
Sample Selection
Medicare patients age 66 years and older with histologically confirmed GBM, newly diagnosed between 2007 and 2013, were retrospectively identified from the database. Patients were followed from GBM diagnosis to death, Medicare disenrollment, health maintenance organization (HMO) enrollment, or December 31, 2014, whichever occurred first. Patients were included in the sample if they were diagnosed with GBM (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] codes: 9440, 9441, 9442) as their primary cancer and with Medicare claims available starting from 12 months prior to diagnosis until the end of their follow-up. Patients were required to be age 66 years or older at the time of diagnosis (ie, 1 year after their Medicare age-based eligibility start) to allow for the 12 months of prediagnosis clinical information, such as baseline comorbidities. Patients were excluded if they 1) had an unknown diagnosis date, 2) received a postmortem GBM diagnosis, 3) had other cancer(s) in the 5 years prior to GBM diagnosis, 4) were not continuously enrolled in Medicare Parts A and B in the 12 months prior to GBM diagnosis, 5) were enrolled in an HMO in the 12 months prior to GBM diagnosis, 6) had more than 1 GBM cancer diagnosis on different dates, or 7) did not receive a diagnostic biopsy or surgery at any point during the study period.
Study Measures and Outcomes
Baseline Characteristics
The following baseline demographics and clinical characteristics were examined: age, sex, race, marital status, census location, urban location, tumor laterality, tumor extension, topographic location, and tumor size. A proxy for poor performance status was created and defined as having a claim indicating a walking aid, oxygen use, wheelchair, or hospice use in the baseline period. Comorbidities were calculated using the Charlson Comorbidity Index (CCI) during the 12-month prediagnosis period. Other comorbidities relevant to GBM but not part of the CCI were also reported, including thromboembolism, pulmonary embolism, Alzheimer disease, epilepsy, cerebral edema, coagulopathy, weight loss, fluid and electrolyte disorders, psychoses, and depression. In addition, the change in CCI and the other comorbidities was examined from baseline to the period following GBM diagnosis to understand the impact of GBM diagnosis on the burden of comorbidities, excluding cancer.
Treatment Patterns
Using Medicare claims data, LOTs were defined according to the following algorithm. The first systemic therapy (1L) was either chemotherapy or bevacizumab with or without concurrent RT use postdiagnosis. A subsequent LOT was defined as: 1) new systemic therapy added more than 30 days after the start date of the prior systemic therapy (if an additional systemic therapy of prior systemic therapy was added within 30 days or less it was considered a combination treatment regimen as part of the original treatment), or 2) the same systemic therapy resumed after a gap of more than 90 days between treatments.28,29 Treatment patterns of patients with GBM were analyzed in terms of the following treatment groups: 1) no cancer-related treatment (defined as no systemic therapy and/or radiation), 2) RT alone, 3) systemic therapies. Systemic therapies were evaluated in terms of the number and duration of LOTs designated as 1L, 2L, and 3 or more lines (3L+) of therapy.
Drugs or regimens were reported by LOT based on the following drug groupings: for 1L, TMZ, bevacizumab, systemic combination therapy, and other chemotherapy monotherapy; for 2L: TMZ, bevacizumab, chemotherapy + bevacizumab, other chemotherapy monotherapy, and chemotherapy combination; and for 3L: TMZ, bevacizumab, other chemotherapy monotherapy, bevacizumab + chemotherapy, and chemotherapy combination. Lastly, time from diagnosis to start of treatment, duration of each LOT, and time to next LOT were reported.
Overall Survival
Survival time was reported using the Kaplan-Meier method and stratified with respect to LOT. For patients who did not receive cancer-related treatment, survival time started on the GBM diagnosis date. For patients who received 1L or more, survival time started on the date of 1L initiation. For patients who received 2L or more, survival time started on the date of 2L initiation. The survival time ended at death or censoring for all LOTs. Median and 1-year survival estimates were reported by age, CCI, and tumor size among patients with GBM who had biopsy or resection.
Health Care Resource Use and Direct Medical Costs
HCRU and costs were reported in 3 distinct time periods relative to diagnosis: prediagnosis (12 months to 3 months before GBM diagnosis), peridiagnosis (3 months before GBM diagnosis), and postdiagnosis (GBM diagnosis month to end of follow-up). HCRU were reported in terms of proportion of patients with each HCRU and mean per-patient-per-month (PPPM) estimates. Costs were reported in terms of mean PPPM and cumulative costs over the follow-up period.
HCRU items that were queried included diagnostics (surgical biopsy, surgical resection, CT scan, and MRI scan of the brain), prescription drugs (antianxiety medication, anticonvulsant, antidepressant, antiemetics, proton pump inhibitor, sedatives/hypnotics, systemic steroids, and narcotic opioids), admissions (emergency room [ER], home health, hospice, hospital admission, ICU, and skilled nursing facility [SNF]), and supportive care (occupational therapy, physical therapy, psychological therapy, and speech therapy).
Costs (reimbursed amounts within Medicare claims) were classified in 2 ways. First, costs were reported by setting, that is, inpatient, outpatient, physician, durable medical equipment, home health agency, hospice, and Part D prescription drugs. Second, line-item costs for certain HCRU items (hospitalizations, surgical resection, surgical biopsy, systemic therapy, hospice stay, RT, MRI scan, ER admission, and CT scan) were reported.
Statistical Analysis
Descriptive statistics were used to evaluate baseline characteristics. For categorical variables, frequency and percentage distributions were reported. For continuous variables, mean, SD, median, and ranges were reported as appropriate. The Kaplan-Meier method for survival analysis was used to describe the time-to-mortality comparing different LOT groups, and the log-rank test was used to determine statistical significance. Median (1-year) survival and P values based on the log rank test were reported. The 95% CIs were reported using the Brookmeyer and Crowley methodology.30 A multinomial logistic model was fit to identify patient factors that were predictive of receipt of RT alone, 1L, 2L, and 3L or more therapy (vs no cancer-related treatment) and odds ratios with 95% CI were reported. HCRU were reported in terms of PPPM estimates in each time period. Mean cumulative costs with bootstrapped 95% CI were reported over the entire study period and in each time period. Data analyses were performed using SAS 9.4 software (SAS Institute Inc). All significance tests were 2-sided, with a P value < .05 considered statistically significant.
Results
Baseline Characteristics
We identified 12 067 patients with histologically confirmed GBM as their primary cancer, diagnosed between 2007 and 2013. Among these, 4308 patients met the inclusion criteria (Supplementary Fig. S1). The median age at diagnosis was 74 years, 54.4% of the patients were male, 63.7% were married, 88.1% were non-Hispanic white, and 43.5% were in the West Census region. Prior to diagnosis 52.0% of patients had a CCI score of 0, and 25.5% had a poor performance proxy indicator (Table 1).
Table 1.
Baseline Characteristics of Full Cohort and by Lines of Therapy
| Patient Characteristics | Category | GBM Cohort (N = 4308) |
|---|---|---|
| Age at diagnosis, n (%), y | 66-70 | 1259 (29.2) |
| 71-75 | 1175 (27.3) | |
| 76-80 | 1021 (23.7) | |
| 80+ | 853 (19.8) | |
| Sex, n (%) | Male | 2344 (54.4) |
| Female | 1964 (45.6) | |
| Race/Ethnicity, n (%) | Non-Hispanic white | 3797 (88.1) |
| Non-Hispanic black | 158 (3.7) | |
| Hispanic | 231 (5.4) | |
| Other | 122 (2.8) | |
| Marital status at diagnosis, n (%) | Single (never married) | 302 (7.0) |
| Married | 2746 (63.7) | |
| Separated/Divorced/Widowed | 1126 (26.1) | |
| Unknown | 134 (3.1) | |
| Census location, n (%) | West | 1872 (43.5) |
| South | 985 (22.9) | |
| Northeast | 905 (21.0) | |
| Midwest | 546 (12.7) | |
| Urban location, n (%) | Rural | 476 (11.0) |
| Urban | 3832 (89.0) | |
| Charlson Comorbidity Index, n (%) | 0 | 2239 (52.0) |
| 1 | 1125 (26.1) | |
| 2 | 492 (11.4) | |
| 3+ | 452 (10.5) | |
| Poor performance status, n (%) | Yes | 1097 (25.5) |
| Laterality, n (%) | Right side | 1873 (43.5) |
| Left side | 1750 (40.6) | |
| Unknown or midline | 685 (15.9) | |
| Tumor extension, n (%) | Supratentorial tumor confined to 1 side | 3069 (71.2) |
| Confined to brain or meninges | 243 (5.6) | |
| Confined to ventricles | 161 (3.7) | |
| Tumor crosses the midline | 596 (13.8) | |
| Unknown | 83 (1.9) | |
| Other | 156 (3.6) | |
| Topographic location of tumor, n (%) | Frontal lobe | 1185 (27.5) |
| Temporal lobe | 1136 (26.4) | |
| Parietal lobe | 729 (16.9) | |
| Overlapping sites | 613 (14.2) | |
| Cerebrum (except lobes) | 395 (9.2) | |
| Occipital lobe | 223 (5.2) | |
| Cerebellum | 26 (0.6) | |
| Tumor size, n (%) | Less than 50 mm | 723 (16.8) |
| Between 50 and 70 mm | 2713 (63.0) | |
| Greater than 70 mm | 210 (4.9) | |
| Missing | 662 (15.4) | |
| Diagnosis year, n (%) | 2007 | 643 (14.9) |
| 2008 | 584 (13.6) | |
| 2009 | 619 (14.4) | |
| 2010 | 592 (13.7) | |
| 2011 | 581 (13.5) | |
| 2012 | 637 (14.8) | |
| 2013 | 652 (15.1) |
Abbreviation: GBM, glioblastoma.
Change in Comorbidity Profile
The median CCI at baseline (in the year prior to GBM diagnosis) was 0 for all patients and increased to 2 in the postdiagnosis period (median follow-up time, 5.6 months postdiagnosis). The drivers of this increase in CCI were paralysis and dementia (Supplementary Table S1). Other comorbidities, not captured in the CCI, that increased significantly from baseline include thromboembolism (0.6% vs 6.8%), Alzheimer disease (1.9% vs 4.0%), epilepsy (8.8% vs 56.5%), cerebral edema (5.9% vs 65.3%), coagulopathy (2.1% vs 18.7%), weight loss (1.8% vs 13.8%), fluid and electrolyte disorders (9.0% vs 48.4%), psychoses (3.6% vs 13.1%), and depression (6.2% vs 21.7%).
Treatment Patterns
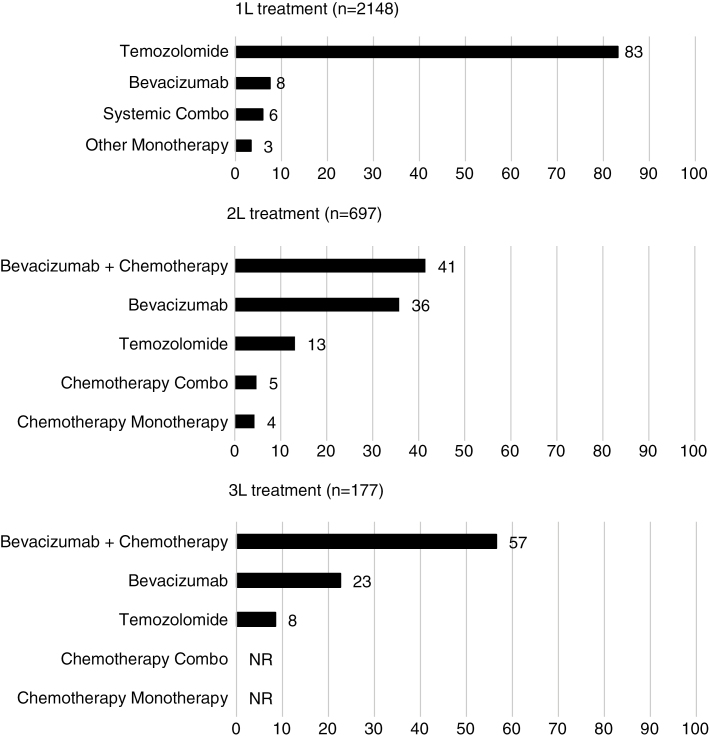
Among the patients in our sample, 2171 (50.4%) received systemic therapy ± RT, 1188 (27.6%) received RT alone, and 949 (22.0%) did not receive any cancer-related treatment (systemic therapy or RT). Among patients with GBM who received systemic therapy, 712 (32.8%) patients received 2L or more, and 183 (25.7%) patients received 3L or more (Supplementary Fig. S2). The most commonly used 1L therapy was TMZ (83.2%). The most commonly used 2L and 3L was chemotherapy + bevacizumab (41.0% of 2L; 56.5% of 3L) followed by bevacizumab (35.7% of 2L; 22.6% of 3L; Fig. 1).
Fig. 1.
Most commonly used treatments at each line of therapy (proportion of patients, %, in each line of therapy). NR indicates not reported because number of patients was either less than 11 or could be used to derive patient numbers less than 11.
Patients with GBM initiated systemic treatment soon after diagnosis (median, 1.5 months). This median time was longer for patients who had resection compared with biopsy (1.1 vs 1.7 months). The median (interquartile range) duration of 1L, 2L, and 3L or more therapies was 2.4 (1.4-5.7), 3.2 (1.4-6.5) and 2.8 (1.4-4.8) months, respectively. The mean (SD) duration of 1L, 2L, and 3L or more therapies was 4.2 (4.5), 4.9 (5.5), and 3.8 (3.7) months, respectively. The median time from end of 1L to start of 2L was 6.4 months, and median time from end of 2L to start of 3L was 5.6 months. Beyond 1L, there was no clear treatment pathway with patients receiving various combinations of systemic therapy (Supplementary Fig. S3).
In terms of predictors of receipt of LOT, patients were more likely to get additional lines if they were younger at diagnosis, married, had a lower CCI score, did not have a poor performance status indicator, or had smaller tumor size at diagnosis (Supplementary Table S3).
Overall Survival
The mOS varied depending on whether patients received surgical resection, number of LOTs, age, CCI, and tumor size (Table 2). Resected patients had a mOS of 5.9 months from diagnosis compared with 3 months for patients who had a biopsy only. In patients without any cancer-related treatment, mOS was similar between resected (2.0) and biopsy (2.6) groups. In patients receiving RT alone, resected patients had a mOS of 3.6 months compared with 2.3 months for patients who had a biopsy. Among patients who received systemic therapy, resected patients had a mOS of 8.8 months from the start of 1L compared with 3.6 months for patients who had a biopsy only. From the beginning of 2L, resected patents had a mOS of 8 months compared with 6.5 months for patients who had a biopsy only. Tumor size, age, and CCI played an important role in the heterogeneity of mOS in resected patients through the first 2 lines of treatment.
Table 2.
Median Overall Survival (1-Year, Survival %) by Lines of Therapy and Surgical Resection/Biopsy
| All | No Cancer-Related Treatment | Radiation Alone | 1L+ | 2L+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 3646) | (n = 748) | (n = 1032) | (N = 1866) | (N = 609) | ||||||
| R | B | R | B | R | B | R | B | R | B | |
| n = 2660 | n = 986 | n = 414 | n = 334 | n = 787 | n = 245 | n = 1496 | n = 370 | n = 530 | n = 79 | |
| Median follow-up time from diagnosis, mo | 7.4 | 3.6 | 2.6 | 2.1 | 5.0 | 3.7 | 11.9 | 5.4 | 18.0 | 13.4 |
| All patients | 5.9 (26) | 3.0 (8) | 2.0 (0) | 2.6 (3) | 3.6 (13) | 2.3 (4) | 8.8 (38) | 3.6 (16) | 8.0 (31) | 6.5 (22) |
| Tumor size (cm) | ||||||||||
| <5 | 6.5 (32)a | 3.6 (14)a | 2.8 (4)a | 2.3 (0)a | 3.7 (17)a | 2.5 (7.4) | 10.1 (43)a | 6.6 (25)a | 8.4 (35) | 8.1 (19) |
| 5-7 | 5.9 (26)a | 3.0 (7)a | 2.7 (4)a | 2.1 (0)a | 3.8 (13)a | 2.3 (3.5) | 8.6 (37)a | 3.2 (14)a | 7.9 (30) | 6.3 (26) |
| >7 | 3.8 (13)a | 2.2 (4)a | 2.0 (0)a | 1.6 (0)a | 2.7 (4)a | 1.9 (0) | 6.0 (26)a | 2.5 (6)a | 6.0 (25) | 1.7 (0) |
| Age, y | ||||||||||
| 66-70 | 8.1 (36)a | 3.1 (11)a | 2.7 (7) | 1.8 (0) | 4.7 (19)a | 3.1 (5)a | 10.3 (44)a | 3.8 (21) | 8.1 (32) | 6.9 (28) |
| 71-75 | 6.4 (27)a | 3.4 (8)a | 2.7 (5) | 2.3 (0) | 4.3 (18)a | 2.9 (7)a | 8.4 (36)a | 3.9 (13) | 8.0 (30) | 6.2 (19) |
| 76-80 | 5.0 (21)a | 3.1 (11)a | 2.6 (0) | 2.0 (0) | 3.2 (10)a | 2.2 (1)a | 7.0 (32)a | 3.9 (23) | 7.3 (29) | 7.6 (21) |
| ≥80 | 4.0 (13)a | 2.8 (4)a | 2.5 (0) | 2.0 (0) | 2.6 (5)a | 2.0 (3)a | 6.6 (31)a | 3.2 (10) | 8.5 (38) | 6.5 (14) |
| CCI | ||||||||||
| 0 | 6.8 (31)a | 3.2 (10) | 2.7 (4) | 2.1 (0) | 4.2 (15)a | 2.8 (5) | 10.1 (43)a | 3.6 (19) | 7.8 (30) | 8.2 (29)a |
| 1 | 5.9 (25)a | 2.8 (6) | 2.5 (0) | 2.1 (0) | 3.4 (13)a | 1.9 (1) | 8.1 (34)a | 3.0 (14) | 8.5 (35) | 3.9 (0)a |
| ≥2 | 4.2 (17)a | 3.1 (6) | 2.4 (4) | 2.0 (0) | 2.7 (9)a | 2.0 (4) | 6.4 (26)a | 4.9 (14) | 7.6 (27) | 4.5 (21)a |
Abbreviations: R, surgical resection; B, biopsy only; CCI, Charlson Comorbidity Index; 1L+, received first line of systemic therapy; 2L+, received second line of systemic therapy. Time starts from diagnosis date from the untreated and from the start of the line in the treated (1L+ starts from start of first line; 2L+ starts from start of second line).
aStatistical significance (P < .05) among categories of tumor size, age, and CCI.
Health Care Resource Use
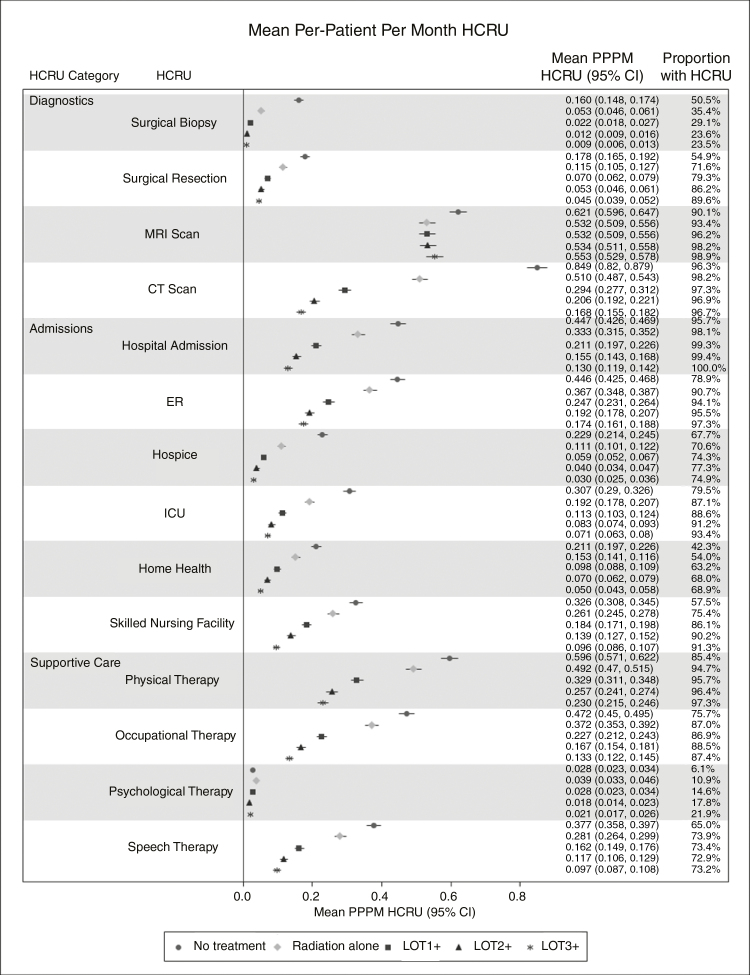
In all patients, 71.8% of patients had a surgical resection and 28.2% had a biopsy (including 7.3% of patients who were coded both for biopsy and surgical resection the same day). Most patients used MRI and CT scans in the postdiagnosis period, with MRI scans being used more frequently on a PPPM basis (0.539 vs 0.379). However, the use of CT scans PPPM was lower because patients received more lines (0.29 for ≥1L, 0.21 for ≥2L, 0.17 for ≥3L). In contrast, PPPM MRI use remained relatively consistent (0.53 for ≥1L, 0.53 ≥ 2L, 0.55 for ≥3L) by the number of lines received, implying that MRIs are used routinely in clinical practice to monitor for progression (Fig. 2).
Fig. 2.
Per-patient-per-month HCRU of diagnostics, admissions, and supportive care in patients with glioblastoma receiving no systemic therapy, 1 or more lines, 2 or more lines, and 3 or more lines. ER indicates emergency room; HCRU, health care resource use; ICU, intensive care unit; PPPM, per-patient-per-month.
The proportion of patients who had an ER admission in the postdiagnosis period was 89.8% in all patients and was highest (97.3%) in patients who received at least 3L and lowest in patients who did not receive any LOT. Many (71.8%) patients went into hospice and most (98%) had a hospitalization in the postdiagnosis period. A great proportion of patients had ICU admission (86.2%), SNF (76.9%), and home health (56.0%) claims in the postdiagnosis period. It is also worth noting that the majority of ICU admissions (85%) and SNF admissions (49%) occurred during the month of diagnosis. Generally, across all admissions, the PPPM use went down as patients received additional LOTs.
In terms of supportive care, in descending order, many patients had physical (93.1%), occupational (84.4%), speech (71.7%), and psychological (11.7%) therapy in the postdiagnosis period. The use of prescription drugs in the postdiagnosis period was also prevalent in our sample. The most commonly used supportive prescription drug class was antiemetics (40.1%) followed by steroids (24.2%). As patients received more LOTs, the proportion of patients using prescription drugs in the postdiagnosis period increased. (Supplementary Table S2).
Direct Medical Costs
The mean cumulative (PPPM) cost for a Medicare GBM patient was $98 710 ($17 800) in the peri- and postdiagnosis periods, of which 58% was incurred in the inpatient setting. Among 1L+ patients, the postdiagnosis mean cumulative (PPPM) cost was $124 138 ($13 041), of which 50% was in the inpatient setting. Patients receiving RT only had lower costs compared with patients receiving more than 1L systemic therapies ± RT ($79 009 vs $124 138; Table 3, Supplementary Fig. S4).
Table 3.
Cumulative Per-Patient and PPPM Costs in US$ (% Total) in the Pre-, Peri, and Postdiagnosis Periods
| Cost Category | Prediagnosis | Peridiagnosis | >Postdiagnosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (n = 4308) | All Patients (n = 4308) | No Cancer-Related Treatment (n = 949) | Radiation Therapy Alone (n = 1188) | 1L+ (n = 2171) | 2L+ (n = 712) | 3L+ (n = 183) | ||||||||||
| Cost | % | Cost | % | Cost | % | Cost | % | Cost | % | Cost | % | Cost | % | |||
| Cumulative Costs | ||||||||||||||||
| Total costs | $4887 | 100 | $3505 | 100 | $50 072 | 100 | $79 009 | 100 | $124 138 | 100 | $165 801 | 100 | $208 665 | 100 | ||
| Inpatient | $2036 | 42 | $2101 | 60 | $40 668 | 81 | $55 414 | 70 | $61 583 | 50 | $67 230 | 41 | $74 987 | 36 | ||
| Outpatient | $736 | 15 | $373 | 11 | $765 | 2 | $6370 | 8 | $22 334 | 18 | $41 580 | 25 | $65 496 | 31 | ||
| Physician | $1028 | 21 | $555 | 16 | $2877 | 6 | $7491 | 9 | $13 159 | 11 | $19 195 | 12 | $23 050 | 11 | ||
| DME | $128 | 3 | $49 | 1 | $184 | 0 | $533 | 1 | $13 106 | 11 | $20 256 | 12 | $25 789 | 12 | ||
| Hospice | $0 | 0 | $0 | 0 | $4373 | 9 | $6096 | 8 | $6617 | 5 | $7260 | 4 | $5695 | 3 | ||
| Home health | $178 | 4 | $116 | 3 | $668 | 1 | $2412 | 3 | $4470 | 4 | $5729 | 3 | $6136 | 3 | ||
| Prescription drugs | $782 | 16 | $311 | 9 | $537 | 1 | $692 | 1 | $2870 | 2 | $4552 | 3 | $7511 | 4 | ||
| PPPM Costs | ||||||||||||||||
| Total costs | $543 | 100 | $1168 | 100 | $21 617 | 100 | $17 760 | 100 | $13 041 | 100 | $9821 | 100 | $8332 | 100 | ||
| Inpatient | $226 | 44 | $700 | 60 | $18 217 | 84 | $13 631 | 77 | $7396 | 57 | $4229 | 43 | $3290 | 39 | ||
| Outpatient | $82 | 14 | $124 | 11 | $293 | 1 | $1108 | 6 | $1798 | 14 | $2238 | 23 | $2617 | 31 | ||
| Physician | $114 | 23 | $185 | 16 | $1231 | 6 | $1445 | 8 | $1341 | 10 | $1301 | 13 | $1162 | 14 | ||
| DME | $14 | 3 | $16 | 1 | $74 | 0 | $109 | 0 | $1266 | 10 | $1128 | 11 | $1003 | 12 | ||
| Hospice | $0 | 0 | $0 | 0 | $1978 | 9 | $1020 | 6 | $604 | 5 | $394 | 4 | $247 | 3 | ||
| Home health | $20 | 4 | $39 | 3 | $190 | 1 | $379 | 2 | $461 | 4 | $355 | 4 | $282 | 3 | ||
| Prescription drugs | $87 | 12 | $104 | 9 | $121 | 1 | $68 | 0 | $175 | 1 | $177 | 2 | $232 | 3 | ||
Abbreviations: 1L, first line; 2L, second line; 3L, third line; DME, durable medical equipment; PPPM, per-patient-per-month.
For all patients, the most costly resources from Medicare’s perspective in terms of PPPM costs postdiagnosis were hospitalizations $10 698, surgical resection $5527, surgical biopsy $2138, systemic therapy $975, hospice stay $891, RT $685, MRI scan $153, CT scan $51, and ER admission $111.
Discussion
This large, real-world analysis of Medicare patients with GBM reveals several key findings. First, only one-half of Medicare GBM patients received at least 1L systemic therapy, and about one-third of those received a subsequent LOT. Second, this analysis also shows that there is heterogeneity in OS, with unresected patients having the worst survival. Although survival improves with the initiation of 1L systemic therapy, median OS remains less than 12 months. Third, GBM is a costly and resource-intensive disease. The mean cumulative costs for a Medicare GBM patient who received 1L systemic therapy was $124 138, of which 50% was incurred in the inpatient setting. Most of the costs were incurred during the month of diagnosis mostly because of inpatient costs (ie, surgical resection) involved at diagnosis. Many (72%) patients went into hospice, and most (98%) had a hospitalization in the postdiagnosis period. The proportion of patients having claims for physical therapy (93%), occupational (84%), and speech (72%) therapy was also high. Lastly, the comorbidity profile of patients with GBM worsens after a diagnosis of GBM, thus adding to the clinical burden of the disease.
The findings from the treatment patterns analysis were consistent with previous reports.22,23,31–33 For example, a previous analysis using the same database found that 29% of patients with GBM did not receive TMZ and/or RT (compared with 28% in our analysis).22 Another analysis in a commercially insured population showed that 59% of patients with GBM did not receive TMZ after brain surgery.23 Our analysis showed that 50% of our sample did not receive any systemic therapy, although results should not be directly compared because the authors of the prior analysis examined only TMZ receipt in a commercially insured patient population whereas the current analysis is specifically focused on Medicare patients with GBM. Future research is needed to explain why 1 in 2 Medicare patients with GBM does not receive any systemic therapy. It will be interesting to see whether the proportion of untreated patients and the patterns of treatments in the real-world setting evolve as more evidence about the role of novel therapies in the overall management of GBM becomes available. Of note, this analysis found that the median duration of 1L (mostly TMZ) therapy was 2.4 months, which is shorter than the conventional (6 cycles of 28 days) or extended (12 cycles of 28 days) adjuvant TMZ regimen. There are 2 potential reasons for this. First, as documented by our analysis, older patients have poor survival and therefore may not live long enough to finish their treatment. Second, a prior analysis has shown that 75% of older patients discontinued adjuvant TMZ therapy because of progression, bone marrow toxicity, or fatigue.34 The same analysis shows that only 25% of older patients get to complete all 6 cycles.34 Similarly, 2 prior analyses in the older population have found the median number of TMZ cycles received were 235 and 4,36 respectively. The median duration of 2L (mostly bevacizumab) therapy was 3.2 months, which is longer than 1L. There could be 2 reasons for this. First, bevacizumab has not been shown to improve survival37,38 and is given mostly for palliation. Second, pseudoresponse from bevacizumab-based therapies could potentially mean that patients stay on bevacizumab longer.39
Survival in GBM has always been poor; however, real-world studies have compared survival rates in the eras pre– and post–TMZ approval and pre– and post–bevacizumab approval. These analyses found that the timing of survival improvement overlapped with the approval of TMZ and bevacizumab.10,40,41 Our survival estimates closely resemble previous reports and prognostic studies.22,32,33
The economic burden of GBM has been examined previously both in commercial and Medicare claims. A prior analysis using commercial claims demonstrated that the average total costs in the 6 months postsurgery were $106 896, ranging from $79 099 for patients who received neither TMZ nor radiation to $138 767 for those who received both therapies.23 In another analysis that used commercial claims, mean total cumulative costs per patient from 3 months prediagnosis to 12 months and to 5 years postdiagnosis were $201 749 and $268 031, respectively.18 In a third analysis by Burton et al that focused on Medicare patients, the mean payer-reported treatment cost following diagnostic surgery for all patients was $60 380, and ranged from $38 600 in patients who did not receive RT or TMZ as initial treatment following diagnostic surgery to $103 762 for patients treated after surgery with RT plus TMZ.22 Our cumulative mean cost estimate for our analysis was higher than Burton and colleagues ($95 377 vs $60 380). There may be 2 possible reasons for this. First, the prior analysis’ timing spanned a period during which bevacizumab was not yet approved (1997 to 2009). Second, the proportion of untreated patients was higher compared with our analysis (29% vs 25%), which will cause the mean cost to increase as more patients receive some form of treatment.
The use of certain supportive prescription drugs in Medicare patients underscored the resource-intensive nature of GBM. Previous reports did not provide consistent estimates around supportive care use.20,42 A 2015 United States–based analysis of chart data in patients who received 1L and 2L therapy reported a somewhat higher proportion of patients receiving prescription drugs compared with our estimates: corticosteroids (85% vs 61%), antiepileptics (51% vs 23%), narcotic opioids (49% vs 35%), proton pump inhibitors (48% vs 8%), and antidepressants (26% vs 16%).20 The Glioma Outcomes Project conducted in academic and community practices in 2005 has reported higher corticosteroid use, antiepileptic use, and lower antidepressant use.42 Owing to the differences in time periods, age groups studied, and data sources, it would be challenging to compare these estimates.
This analysis has several key strengths. First, it used a nationally representative database that represents the older US population with GBM. Second, our analysis is unique in that it provided a range of survival estimates that represented the heterogeneity of GBM survival outcomes. Third, this analysis estimated the costs of common health care resources used by patients with GBM and quantified the costs incurred, identifying the major cost drivers. Previous analyses using commercial claims focused on characterizing HCRU and costs among TMZ-treated patients.18,23 Our study, on the other hand, has also quantified the economic burden of patients who did not receive systemic therapy, which represents one-half of Medicare patients presenting with GBM. This was possible because we used the ICD-O-3 coding system available from SEER (as opposed to the nonspecific ICD-9-CM coding system available in claims). Fourth, because costs for GBM treatment were established in the literature, we wanted to describe the resources used by these patients relative to their diagnosis from a payer’s perspective. Our analysis is unique in that we observed a significant amount of costs incurred before patients were diagnosed with GBM.
Some of the potential limitations of the analysis include the possibility of misclassification of treatments when defining LOT. This analysis used Medicare payer claims, which are generated for the purposes of provider reimbursement and not necessarily for research purposes. For example, a combination of algorithms and clinical expertise was used to define LOT and as a result, certain patients may have been misclassified. Similarly, use of claims to define clinical events are not ideal. For example, the increased Alzheimer disease from pre- to postdiagnosis may be miscoded as cognitive decline related to therapy and/or tumor. In addition, some of the results presented here may not be generalizable to younger patients with GBM given that the population studied was younger than 66 years. Additionally, O6-methylguanine–DNA methyltransferase (MGMT) promoter methylation was not analyzed because it was not available in the dataset.43MGMT promoter methylation has been associated with longer survival in patients with GBM who receive TMZ. It is a major consideration in treatment decision making based on NOA-08, NORDIC, and post hoc analyses of the Stupp and Perry trials.17,44–46 Lastly, we were able to highlight treatment patterns only through December 2014, the latest year of data availability at the time of the analysis.
As the GBM treatment landscape evolves with the initiation of clinical trials to study novel treatment options, this analysis can provide a benchmark for the current standards of care and relative costs associated with GBM treatment across different lines of systemic therapy in the United States. The limited treatment options, poor survival outcomes, and substantial economic burden demonstrate the importance of identifying new innovative treatment options for patients with GBM, especially for the older population.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by Bristol-Myers Squibb (Princeton, NJ).
Conflict of interest statement.
AA and YLL were employees of Pharmerit International during this study. MB and HPK are current employees of Pharmerit International; MFB is also a shareholder of Pharmerit International. PS, BK, Y-LL, and HD are employees of and have stock options in BMS. AN is an employee of and has stock options in Cota Healthcare, Inc, New York, NY.
Acknowledgments
Two posters using an older version of the data were presented at the American Society of Clinical Oncology and International Society for Pharmacoeconomics and Outcomes Research conferences in 2017. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute (NCI)’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention (CDC)’s National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California Department of Public Health, the NCI, and CDC or their contractors and subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the NCI; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thakkar JP, Dolecek TA, Horbinski C, et al. . Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004;101(10):2293–2299. [DOI] [PubMed] [Google Scholar]
- 4. Gittleman H, Lim D, Kattan MW, et al. . An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro Oncol. 2017;19(5):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 6. Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2(4):386–393. [PMC free article] [PubMed] [Google Scholar]
- 7. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY,Olson JJ.. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balmaceda C, Peereboom D, Pannullo S, et al. . Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer. 2008;112(5):1139–1146. [DOI] [PubMed] [Google Scholar]
- 9. Polivka J, Polivka J Jr, Krakorova K, Peterka M, Topolcan O.. Current status of biomarker research in neurology. EPMA J. 2016;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. [DOI] [PubMed] [Google Scholar]
- 11. Stupp R, Wong ET, Kanner AA, et al. . NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. doi:10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 12. Wick W, Puduvalli VK, Chamberlain MC, et al. . Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taal W, Oosterkamp HM, Walenkamp AM, et al. . Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 14. Brandes AA, Franceschi E, Tosoni A, et al. . Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115(15):3512–3518. [DOI] [PubMed] [Google Scholar]
- 15. Roa W, Brasher PM, Bauman G, et al. . Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 16. Roa W, Kepka L, Kumar N, et al. . International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
- 17. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 18. Jiang S, Hill K, Patel D, et al. . Direct medical costs of treatment in newly-diagnosed high-grade glioma among commercially insured US patients. J Med Econ. 2017;20(12):1237–1243. [DOI] [PubMed] [Google Scholar]
- 19. Raizer JJ, Fitzner KA, Jacobs DI, et al. . Economics of malignant gliomas: a critical review. J Oncol Pract. 2015;11(1):e59–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Girvan AC, Carter GC, Li L, et al. . Glioblastoma treatment patterns, survival, and healthcare resource use in real-world clinical practice in the USA. Drugs Context. 2015;4:212274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Messali A, Villacorta R, Hay JW. A review of the economic burden of glioblastoma and the cost effectiveness of pharmacologic treatments. Pharmacoeconomics. 2014;32(12):1201–1212. [DOI] [PubMed] [Google Scholar]
- 22. Burton E, Ugiliweneza B, Woo S, Skirboll S, Boaky M.. A Surveillance, Epidemiology and End Results-Medicare data analysis of elderly patients with glioblastoma multiforme: treatment patterns, outcomes and cost. Mol Clin Oncol. 2015;3(5):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ray S, Bonafede MM, Mohile NA. Treatment patterns, survival, and healthcare costs of patients with malignant gliomas in a large US commercially insured population. Am Health Drug Benefits. 2014;7(3):140–149. [PMC free article] [PubMed] [Google Scholar]
- 24. Snyder J, Walbert T. Managing glioblastoma in the elderly patient: new opportunities. Oncology (Williston Park). 2017;31(6):476–483. [PubMed] [Google Scholar]
- 25. Puduvalli VK, Chaudhary R, McClugage SG, Markert J.. Beyond alkylating agents for gliomas: quo vadimus? Am Soc Clin Oncol Educ Book. 2017;37:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noone AM, Lund JL, Mariotto A, et al. . Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54(9):e55–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG.. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–748. [PubMed] [Google Scholar]
- 28. Bikov KA, Mullins CD, Seal B, Onukwugha E, Hanna N.. Algorithm for identifying chemotherapy/biological regimens for metastatic colon cancer in SEER-Medicare. Med Care. 2015;53(8):e58–e64. [DOI] [PubMed] [Google Scholar]
- 29. Liang C, Li L, Fraser CD, et al. . The treatment patterns, efficacy, and safety of nab®-paclitaxel for the treatment of metastatic breast cancer in the United States: results from health insurance claims analysis. BMC Cancer. 2015;15:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang S, Jeong JH. Median tests for censored survival data; a contingency table approach. Biometrics. 2012;68(3):983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amsbaugh MJ, Yusuf MB, Gaskins J, Burton EC, Woo SY.. Patterns of care and predictors of adjuvant therapies in elderly patients with glioblastoma: an analysis of the National Cancer Data Base. Cancer. 2017;123(17):3277–3284. [DOI] [PubMed] [Google Scholar]
- 32. Arrigo RT, Boakye M, Skirboll SL. Patterns of care and survival for glioblastoma patients in the Veterans population. J Neurooncol. 2012;106(3):627–635. [DOI] [PubMed] [Google Scholar]
- 33. Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE.. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 34. Lorimer CF, Hanna C, Saran F, Chalmers A, Brock J.. Challenges to treating older glioblastoma patients: the influence of clinical and tumour characteristics on survival outcomes. Clin Oncol (R Coll Radiol). 2017;29(11):739–747. [DOI] [PubMed] [Google Scholar]
- 35. Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. . Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. [DOI] [PubMed] [Google Scholar]
- 36. Biau J, Chautard E, De Schlichting E, et al. . Radiotherapy plus temozolomide in elderly patients with glioblastoma: a “real-life” report. Radiat Oncol. 2017;12(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 38. Gilbert MR, Dignam JJ, Armstrong TS, et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;76(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson DR, Omuro AMP, Ravelo A, et al. . Overall survival in patients with glioblastoma before and after bevacizumab approval. Curr Med Res Opin. 2018;34(5):813–820. [DOI] [PubMed] [Google Scholar]
- 41. Zhu P, Du XL, Lu G, Zhu JJ.. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: a population-based study. Oncotarget. 2017;8(27):44015–44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang SM, Parney IF, Huang W, et al. ; Glioma Outcomes Project Investigators Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. [DOI] [PubMed] [Google Scholar]
- 43. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 44. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 45. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.