Abstract
Background
It has been suggested that lack of ongoing registration of patient-centered outcomes has resulted in existing care trajectories that have not been optimized for sequelae experienced by meningioma patients. This study aimed to evaluate the structure of current meningioma care and identify issues and potential high-impact improvement initiatives.
Methods
Using the grounded theory approach, a thematic framework was constructed based on the Dutch Comprehensive Cancer Organisation survey about issues in meningioma care trajectories. This framework was used during 3 semistructured interviews and 2 focus groups with patient-partner dyads (n = 16 participants), and 2 focus groups with health care providers (n = 11 participants) to assess issues in current meningioma care trajectories and possible solutions, including barriers to and facilitators for implementation.
Results
Identified issues (n = 18 issues) were categorized into 3 themes: availability and provision of information, care and support, and screening for (neurocognitive) rehabilitation. A lack of information about the intervention and possible outcomes or complications, lack of support after treatment focusing on bodily and psychological functions, and reintegration into society were considered most important. Sixteen solutions were suggested, such as appointment of case managers (solution for 11/18 issues, 61%), assessment and treatment by physiatrists (22%), and routine use of patient-reported outcome measures for patient monitoring (17%). Barriers for these solutions were lack of budget, capacity, technology infrastructure, and qualified personnel with knowledge about issues experienced by meningioma patients.
Conclusions
This study identified issues in current multidisciplinary meningioma care that are considered unmet needs by patients, partners, and health care providers and could guide innovation of care.
Keywords: case manager, meningioma, patient-reported outcome measures (PROMs), value-based health care
Meningiomas are tumors developing from the leptomeninges, accounting for 36.4% of primary intracranial tumors.1–3 More than 80% of meningiomas are benign (World Health Organization [WHO] grade I), and patients have a near-normal life expectancy.2,4 Morbidity is due to compression of the CNS and/or cranial nerves and vessels.4,2 Recent European and Dutch guidelines advise a wait-and-scan policy in patients with asymptomatic meningiomas, and surgery or stereotactic radiotherapy in case of symptoms or established tumor growth.5 Even though their life expectancy is near normal, the limited data currently available suggest that patients suffer from long-term neurological sequelae and that their health-related quality of life (HRQOL) is impaired in all domains compared with the general population, both before and after interventions.6
Meningioma literature and guidelines traditionally focus on the extent of tumor resection, recurrence, and neurological outcomes.5 Though these outcomes are highly relevant, they fail to reflect the continuing impact of the tumor and treatment on a patient’s daily life.7 Owing to the lack of HRQOL data and other patient-reported outcomes, the few existing current care trajectories have not been optimized for these long-term sequelae.5,6 This is supported by recent results from a patient survey in meningioma patients conducted by the Dutch Comprehensive Cancer Organisation (DCCO), which showed that patients experience various problems and unmet needs during their care trajectory, such as a lack of information on treatment and outcomes and lack of meningioma-specific care, for example, meningioma-specific rehabilitation after intervention.8 Although the results of the DCCO survey provided insight into the magnitude of the problem on a national level, the survey lacked detailed information on the actual experienced issues and possible solutions needed to improve current care trajectories.
Because we are in the process of reorganizing meningioma care, we investigated in detail the current state of meningioma care trajectories, particularly focusing on issues that were perceived as problematic. We also studied possible solutions for the identified issues as perceived by patient-partner dyads and health care providers. In addition, we aimed to assess barriers to and facilitators for the implementation of proposed solutions that might have a high impact on the outcomes of meningioma care trajectories as perceived by health care providers.
Materials and Methods
Sampling of Patients, Partners, and Health Care Providers From Meningioma Care Trajectories
In the Netherlands, meningioma care is primarily organized in academic and a few large teaching hospitals. Asymptomatic patients are followed by a neurologist and in case of symptom development or evident tumor growth, patients are referred through a tumor board to a neurosurgeon or radiation oncologist. After intervention, most patients are again followed by a neurologist or in select cases an endocrinologist. Before and after an intervention, some patients are seen by an ophthalmologist, endocrinologist, or health care providers from another specialty (eg, physiatrist) depending on tumor localization and symptoms.
Patients with a clinical suspicion or histopathological confirmation of a WHO grade I or II meningioma, during wait-and-scan follow-up or after surgery or radiotherapy followed at the Leiden University Medical Center (LUMC) or Haaglanden Medical Center (HMC) between November 2017 and April 2018, were invited to participate in this study. Purposive sampling was used to ensure patients were included from all possible care trajectories, that is, based on intervention (surgery, radiotherapy, or wait-and-scan) and follow-up by a neurologist or endocrinologist. In addition, they were included based on their sociodemographic and clinical characteristics, such as age, sex, and tumor location (convexity vs skull base) to ensure generalizability of the study sample toward the general meningioma population.9 Only patients with at least 4 months of follow-up after receiving their last treatment (surgery, radiotherapy) or after initiation of a wait-and-scan follow-up were selected to ensure that patients had experienced a large part of a meningioma postdiagnostic care trajectory. Additional inclusion criteria were age 18 years or older, and adequate Dutch-language skills. Partners were eligible if they had accompanied the patient to his or her appointments on a regular basis. Informed consent was obtained on paper before study participation.
Eligible health care providers were neurosurgeons, neurologists, ophthalmologists, radiation oncologists, psychologists, endocrinologists, and physiatrists, who treated a minimum of 5 new meningioma patients per year and worked at or were affiliated with a Dutch meningioma intervention center.
Study Design and Concept
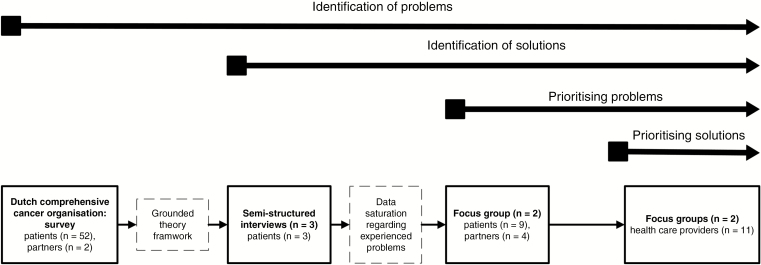
This study consisted of 4 consecutive steps, including data analysis from the DCCO survey (step 1) and semistructured interviews and focus groups (steps 2-4) and was approved by the medical ethics committees of both the LUMC and HMC institutions. Details on the study concept and design are presented in Fig. 1. General procedures for all 4 steps are described in Supplementary Text 1.
Fig. 1.
Study Concept and Design
Step 1: Dutch Comprehensive Cancer Organisation survey
Two researchers independently identified issues from data from the DCCO survey, which were used to construct a thematic framework of issues for each part of the Dutch meningioma care trajectory as identified by meningioma patients (Supplementary Table 1).9 The thematic framework was constructed following the principles of the grounded theory approach, which is an inductive method through which theoretical insights are generated from collected data rather than being restricted by existing theoretical frameworks.10 Detailed information on the patient population cannot be provided because the DCCO survey collected data anonymously. During both the semistructured interviews and focus groups, the whole meningioma care trajectory was discussed and for each part of the care trajectory the relevant themes as described in the thematic framework were discussed (Supplementary Table 1).
Step 2: Semistructured interviews with patients
Separate semistructured interviews were conducted with 3 patients. Using the thematic framework from step 1, participants were asked to identify issues regarding their meningioma care trajectory, as well as possible solutions for these issues. This was performed until data saturation was reached, which was defined as the point at which no new issues were brought up.9,10
Step 3: Focus groups with patient-partner dyads
Two focus group sessions (n = 6 and n = 7 participants) were organized with patients and their partners in an effort to generate possible solutions for issues reported during the semistructured interviews and to evaluate previously reported solutions. The issues were prioritized based on importance at the end of each session.
Step 4: Focus groups with health care providers
Two focus group sessions (n = 5 and n = 6 participants) were organized with health care providers, aiming at identifying potential solutions for issues reported by patient-partner dyads from a health care provider’s perspective, as well as more details on the raised issues and possible solutions. Through an elaborate process, solutions were prioritized using an adapted Eisenhower matrix, according to the perceived importance and degree of effort (both: high vs low) at the end of each session. In addition, participants were asked to identify barriers to and facilitators for high-importance, high-effort solutions.
Qualitative Analysis of Semistructured Interviews and Focus Groups
Results of the semistructured interviews and focus group sessions were transcribed verbatim and anonymously analyzed by 2 researchers (AZN and JvdM) independently in a 3-step approach, as described in previous studies.11 In step 1, meaningful units were identified, which were allocated to subconcepts in step 2 and grouped into comprehensive concepts in step 3 (an example is given in Supplementary Figure 1). Discrepancies between the 2 researchers were discussed after each step and when no consensus was reached, a third researcher (LD) mediated the discussion. Issues reported as important in at least 2 focus groups or semistructured interviews are reported.
Barriers and facilitators were separated into 6 categories, using the well-established framework of Grol and Wensing, which consists of the following categories: innovation, individual professional, patient, social context, organizational context, and external environment (political and economic factors).12
Reporting was undertaken according to the Consolidated Criteria for Reporting Qualitative Research.13
Quantitative Analysis
Baseline sociodemographic and clinical characteristics are reported for patients, partners, and health care providers separately. Continuous data are reported as medians with an interquartile range because of the small number of participants and the skewed distribution of variables. Nominal data are reported as proportions. All statistics were performed using IBM SPSS Statistics for Windows version 23.0.
Results
In total, 52 patients and 2 partners completed the DCCO survey after a median of 66 months (range, 6-148 months). In addition, 12 patients, 4 partners, and 11 health care providers participated in the semistructured interviews and focus groups. Demographic information on the participants of the semistructured interviews and focus groups is presented in Table 1. Most of these patients were surgically treated (n = 11, 92%) and 4 (25%) patients had also received radiotherapy. Median lengths of follow-up after the last intervention was 24 months (range, 4-148 months). Postoperative complications occurred in 2 patients, namely ischemic stroke of the temporal lobe with transient aphasia and transient deterioration of visual acuity.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Patients, Partners and Health Care Providers Included in Focus Groups and Semistructured Interviews
| Patients (n = 12) |
Partners (n = 4) |
Health Care Providers (n = 11) |
|
|---|---|---|---|
| Age at interview, median (range), y | 52 (39-70) | 56 (47-65) | 42 (39-53) |
| Sex, n (%) female | 10 (83%) | 0 (0%) | 6 (55%) |
| Highest obtained educational degree, n (%) | |||
| Primary/Secondary | 0 (0%) | 0 (0%) | – |
| Vocational/Technical | 5 (42%) | 1 (25%) | – |
| Academic/University | 7 (58%) | 3 (75%) | – |
| Paid job, n (%) | 9 (75%) | 3 (75%) | – |
| Tumor location, n (%) | |||
| Convexity | 4 (25%) | – | – |
| Skull base | 8 (75%) | – | – |
| KPS, median (range) | 100 (50-100) | 100 (100-100) | – |
| Charlson Comorbidity Index, median (range) | 1 (0-7) | 1 (0-1) | – |
| Surgery, n (%) | 11 (92%) | – | – |
| Radiotherapy, n (%) | 4 (25%) | – | – |
| Months since last intervention, median (range) | 24 (4–148) | ||
| Neurological deficits, n (%) | 1 (8%) | – | – |
| Visual deficits, n (%) | 1 (8%) | – | – |
| Academic hospital, n (%) | 8 (68%) | 2 (50%) | 9 (82%) |
| Experience, median (range), y | – | – | 9 (8-20) |
| Average number of new meningioma patients seen each year, median (range) | – | – | 20 (10-25) |
Issues and Solutions
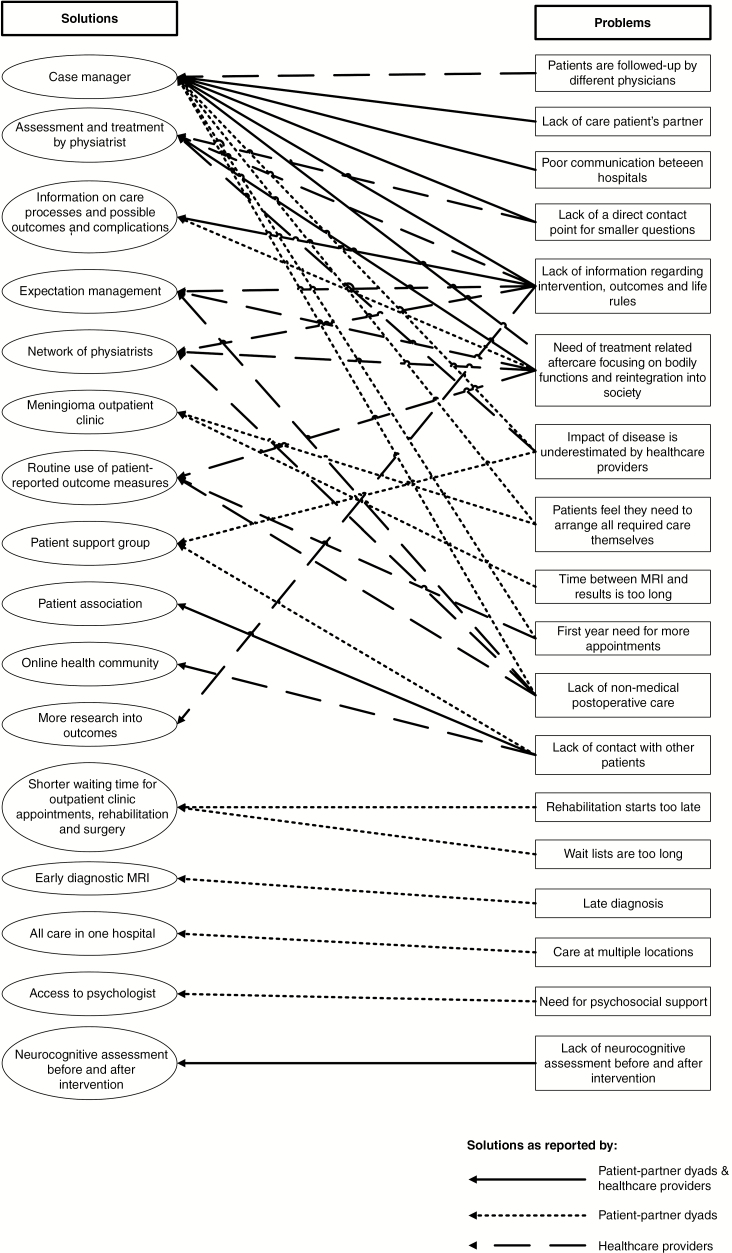
Following the principles of the grounded theory approach, issues were eventually categorized into a thematic framework consisting of the following 3 themes: (1) availability and provision of information, (2) care and support, and (3) screening for (neurocognitive) rehabilitation. A complete overview of all issues and possible solutions is presented in Fig. 2 and Supplementary Table 2. Data saturation on the identified issues was reached after the semistructured interviews, so the focus groups primarily focused on evaluating these issues in more detail and identifying possible solutions for these problems (Fig. 1). Examples of quotes from participants in the semistructured interviews and focus groups are presented in Supplementary Text 2.
Fig. 2.
Issues and Solutions in Meningioma Care Trajectories
Availability and provision of information
Patient-partner dyads and health care providers both reported the following issues as important: 1) not receiving sufficient information about the logistics of care during the period prior to the intervention (surgery or radiotherapy), 2) a lack of information about the intervention itself and what to expect afterward, including information on complications and symptoms, and 3) what they are allowed to do after the intervention (patient quote: “How will I feel after the surgery? And how long will it take to have a somewhat normal life again?”).
A potential solution for these unmet needs was the availability and provision of information (eg, flyer, website) on the care trajectories, treatment options, short-term and long-term outcomes, and potential complications, as suggested both by patient-partner dyads and health care providers. Patient-partner dyads who had positive experiences with guidance from case managers for their comorbidities suggested that a specialized nurse or case manager could potentially provide this information. Health care providers confirmed the necessity; however, they also indicated that more outcome research is necessary to provide evidence-based information on outcomes.
Care and support
Patient-partner dyads and health care providers both reported that patients experience a lack of support, especially in the long-term, by health care providers after being diagnosed and treated for a meningioma. Specifically, information was lacking on 1) bodily functions, 2) reintegration into society, 3) psychosocial aftercare, and 4) care for the partner of the patient (patient quote: “If I only had someone during the process to call to ask questions to, such as whether it’s normal to be so tired the entire day, […] or whether I was allowed to cycle […] I had no idea of what I was capable of doing”). Patient-partner dyads reported the need for a contact person to ensure continuity of care and for minor everyday questions. They furthermore reported they missed having a patient support group and believed that the overall impact of the disease is often underestimated by health care providers. Patient-partner dyads and health care providers both reported that a specialized nurse or case manager could be of assistance to inform and guide patients and their partners after an intervention. Psychological aftercare provided by a specialized health care provider focusing on cognitive revalidation, self-management strategies, and mood disorders such as anxiety and depression is also currently missing, according to patient-partner dyads. In addition, patient-partner dyads expressed the wish for shorter waiting lists for scans, outpatient clinic appointments, and intervention.
Screening for (neurocognitive) rehabilitation
Patient-partner dyads reported the need for a neurocognitive assessment and health care providers the use of patient-reported outcome measures (PROMs) both before and after the treatment to provide patients information about the impact of treatment and the possible need for (neurocognitive) rehabilitation. Health care providers and patient-partner dyads reported needing to have the possibility to refer more patients to a physiatrist to determine whether rehabilitative treatment should be initiated focusing on neurological and physical functions (patient quote: “Fair enough, I received some exercises in the hospital the first 2 weeks, but after that, there was nothing. I did not know what I had to do at all”).
Prioritization and Implementation of Solutions
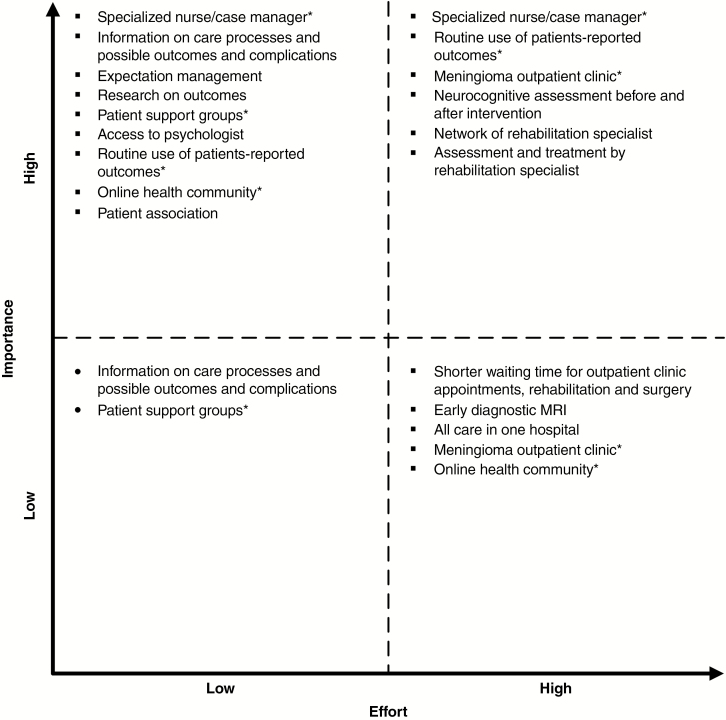
A total of 16 solutions were identified during all the focus groups. Potential solutions for most of the problems could be the appointment of a case manager in current care trajectories (solution for 11/18 problems, 61%), assessment and treatment by a physiatrist (22%), routine use of PROMs (17%), and providing expectation management (17%). Most solutions (56%) were categorized by at least 1 participant as highly important, low-effort solutions that should readily be implemented, for example, access to a (neuro)psychologist and the availability and provision of information on interventions and outcomes of treatments (Fig. 3). High-importance, high-effort solutions (38%) were incorporation of a case manager in current care trajectories, creating a meningioma-specific outpatient clinic, performing neurocognitive assessments before and after an intervention, routine use of PROMs, and routine assessment of the need for rehabilitative therapy by a physiatrist, preferably in a network of physiatrists. The most important barriers for implementing these solutions were a lack of budget, capacity, information and communications technology (ICT) infrastructure, qualified personnel with knowledge about the management of meningioma patients, and treatment issues focusing on HRQOL (Table 2). The most important identified facilitators were using examples from other diseases and hospitals, and prioritization by the hospital board. Most barriers and facilitators could be classified according to the Grol and Wensing criteria12 as factors associated with organizational aspects or the innovation (solution) itself.
Fig. 3.
Adapted Eisenhower Matrix Categorizing Solutions Based on Their Importance/Effort Ratio
Table 2.
Barriers and Facilitators for High-Effort, High-Importance Solutions
| Solution | Barrier (Category) | Facilitator (Category) |
|---|---|---|
| Case manager | • Lack of qualified personnel (organization context) • Multidisciplinary meningioma care (organization context) • Lack of capacity (organization context) • Lack of budget (economic and political context) • Training of nurses (individual professional) |
• Qualified personnel (organization context) • Interdisciplinary consultation by case manager (organization context) • Budget (economic and political context) • Use examples from other diseases (innovation) • Saves time of doctors (innovation) • Results in improvement in quality of care (innovation) • Priority hospital/board of directors (social context) |
| Routine use of patient-reported outcomes | • Lack of time (organization context) • Lack of link with electronic patient record (organization context) • Lack of ICT infrastructure (organization context) • Implementation problems (organization context) • Lack of budget (economic and political context) • Nonvalidated PROMs (innovation) • Unmotivated patients to complete PROMs (patient) |
• Qualified ICT team (organization context) • Link with electronic patient record (organization context) • Use examples from other diseases (innovation) • Use of tablets (innovation) • Well-developed and validated PROMs (innovation) • Motivated patients to complete PROMs (patient) |
| Meningioma outpatient clinic | • Lack of capacity (organization context) • Lack of space and equipment (organization context) • Lack of budget (economic and political context) • Heterogeneity disease (patient) |
• Budget (economic and political context) • Results in publicity for hospital (innovation) • Results in higher patient numbers (innovation) • Results in improvement in quality of care (innovation) • Patient association voicing the need (patient) • Priority hospital/board of directors (social context) |
| Neurocognitive assessment | • Lack of qualified personnel (organization context) • Lack of capacity neuropsychologist (organization context) • Lack of budget (economic and political context) |
• Incorporation of reimbursement system (organization context) • Link with electronic patient record (organization context) • Budget (from board of directors) (economic and political context) • Simultaneous use of data for research (innovation) • Inform patients of usability of neurocognitive assessment (patient) |
| Physiatrist network | • Lack of budget (economic and political context) • Unfamiliarity of other disciplines with rehabilitation possibilities (individual professional) • Lack of know-how (individual professional) • Lack of interest by other disciplines (individual professional) |
• Physiatrist part of multidisciplinary team (organization context) • Budget (economic and political context) • Results in improvement in quality of care (innovation) • Priority hospital/board of directors (social context) |
| Physiatrist screening | • Lack of capacity (organization context) • Lack of budget (economic and political context) • Choice of screening instrument (innovation) |
• Budget (economic and political context) • Results in improvement in quality of care (innovation) • Patient self-screening (patient) • Priority hospital/board of directors (social context) |
Abbreviations: ICT, information and communications technology; PROM, patient-reported outcome measures.
Barriers and facilitators are categorized following the framework of Grol and Wensing into 6 categories: (1) innovation, (2) individual professional, (3) patient, (4) social context, (5) organizational context, and (6) external environment (political and economic factors).12
Discussion
This study identifies issues in current multidisciplinary meningioma care that are considered unmet needs by patients, partners, and health care providers that potentially contribute to delivering suboptimal care. This is the first study systemically evaluating these needs, including the identification of potential high-impact solutions to improve care.
Transition of Care
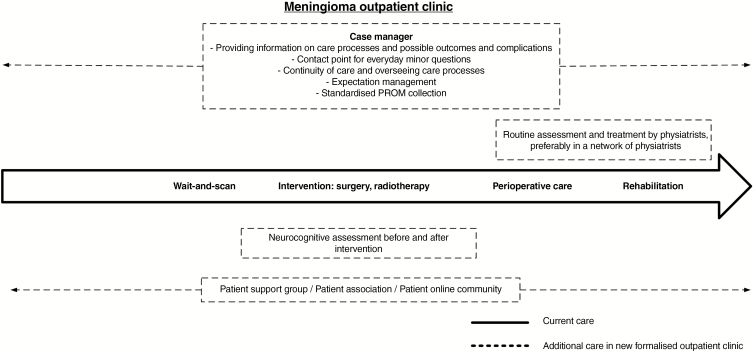
In our tertiary referral center, multiple initiatives have been introduced in recent years to improve the care for patients with skull base and intracranial lesions. For those developing endocrine dysfunction or ophthalmological deficits, a formalized care trajectory was developed, including appointment of dedicated nurse case managers, standardized outcome measurements with PROMs, and implementation of self-management interventions, which all generally showed improvement of care outcomes.14–17 Results of our study strongly support the need for a similar transformation of the care and support system for meningioma patients, as depicted in Fig. 4. Particularly, patients and health care providers reported the need for availability and provision of information about the intervention and its possible outcomes and complications, (continuity) of aftercare for patients and their partners including PROM use, focusing on bodily and psychological functions and reintegration into society, a point of contact for smaller, nonmedical questions, and patient support groups. Addressing these issues may possibly contribute to increased quality of care as well as clinical outcomes. Although physicians may be able to provide this needed extra guidance and aftercare, a nurse case manager seems more time-effective and cost-effective, thereby facilitating value-based meningioma health care.18 Furthermore, to ensure high-quality care on a national level, quality criteria for meningioma centers should be defined regarding the structure of care, minimum number of operations, and routine collection of outcomes. These criteria already exist for other intracranial pathologies such as glioma and pituitary tumors, and have even resulted in the appointment of centers of excellence.19,20
Fig. 4.
Transformation of Meningioma Care.
Evidence for Suggested Solutions
Multiple studies in meningioma and other patient groups have found that the use of nurse case managers, (cognitive) rehabilitation programs, and routine assessment with PROMS in care trajectories have led to better outcomes,21–27 and that patients and physicians reported high satisfaction with provided care and perceived improvement in quality of care after appointment of a case manager.28 Though in general the effects were perceived as beneficial, large efforts needed to be made in the beginning to ensure proper implementation of these initiatives. Multiple effective meningioma or intracranial tumor rehabilitation programs exist focusing on bodily functions, cognitive rehabilitation, and self-management.14,24,26 Additionally, there are currently ongoing efforts to develop meningioma-specific PROMS and outcome sets.6,7,29 Though routine assessment with PROMs might be perceived as a burden in effort and time, it is beneficial for patient-doctor communication, adequate monitoring of treatment response (eg, from a patient’s home), reduction of the number of outpatient visits, detection of unrecognized symptoms by physicians, and consequently changes in the treatment and care of patients.30 In general, future studies are needed to assess the actual effect of the suggested solutions on patient’s HRQOL and the additional costs for the care trajectories.
Strengths and Limitations of This Study
A strength of this study is the combination of quantitative and qualitative data, as new issues were identified during the semistructured interviews and focus groups that were not mentioned in the DCCO survey data. Another strength is the inclusion of not only patients, but also partners and health care providers to cover all relevant themes in current meningioma care trajectories. The absence of nurses during the semistructured interviews and focus groups is a limitation because they could have identified different issues and solutions. Through purposive sampling, an adequate representation of meningioma patients was ensured, and health care providers represented almost all specialties involved in meningioma care trajectories. Obviously, like in comparable studies, we could not completely exclude some selection bias because it is likely that only patients, and possibly also health care providers with an interest in this disease and topic, were more likely to participate. Data saturation was reached early in the study process, likely because of the availability of the quantitative results of the DCCO survey. Furthermore, even though we included patients both from an academic and a nonacademic hospital, we were able to include only patients from a specific region in the Netherlands for the semistructured interviews and focus groups, potentially limiting generalizability. However, we were able to include health care providers working in different regions of the Netherlands, which is a strength of the study. Although not all results may be generalized to countries other than the Netherlands, evidence for many of the reported issues and solutions are supported by the international literature.21–23,26,27,31–35 A difficulty with qualitative studies is that commonly only issues—not possible solutions—are identified, hampering actual change of care. Therefore, we asked health care providers to prioritize the identified solutions based on their perceived importance/effort ratio and to identify barriers to and facilitators for implementation of these solutions, which is another strength of this study. Patients were not asked to identify barriers and facilitators for the identified solutions because we felt that a thorough understanding of Dutch hospitals and the Dutch health care system was needed for this purpose. Finally, because the median follow-up of patients was 5.5 years for the DCCO survey and 2 years for the semistructured interviews and focus groups, our results cover the periods around diagnosis and intervention as well as the longer-term sequelae.
Recommendations and Future Directions
In conclusion, the most important issues as identified through patient-partner dyads were a lack of information about the intervention and its possible outcomes and complications, a lack of support after treatment focusing on bodily and psychological functions, and reintegration into society. To improve most of these unmet needs of patients, partners, and health care providers, it is advisable to appoint a case manager, routinely use PROMs, and to incorporate a (neurocognitive) rehabilitation screening program into current meningioma care trajectories. These solutions might subsequently result in lower costs and better outcomes, which is in line with the principles of value-based health care. Information on the identified barriers and facilitators should be used to successfully implement these initiatives. Ideally, these initiatives should be evaluated within integrated practice units (IPUs), which involve the entire multidisciplinary team around the patient group of interest, to ensure broad support.36 Because it is difficult to reach sustainable change in existing care trajectories, iterative evaluation of implemented initiatives is required. For instance, the Plan-Do-Study-Act cycle could be used, which requires initiatives to be redirected based on evaluated outcomes.37,38 Within our IPU, we are currently training case managers and developing a core outcome set together with and for meningioma patients as a first step to reorganize our care following value-based health care principles.
Funding
This work was supported by the Leiden University Medical Center MD/PhD Scholarship and a personal grant by Bewustzijnsproject Nederland [185502563 to AHZN].
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We would like to acknowledge prof Thea P.M. Vliet Vlieland for her input in the study design and prof Saskia M. Peerdeman for her involvement in data collection for the Dutch Comprehensive Cancer Organisation survey.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whittle IR, Smith C, Navoo P, Collie D.. Meningiomas. Lancet. 2004;363(9420):1535–1543. doi: 10.1016/S0140-6736(04)16153-9. [DOI] [PubMed] [Google Scholar]
- 3. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Alkemade H, de Leau M, Dieleman EM, et al. . Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14(5):658–666. doi: 10.1093/neuonc/nos013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldbrunner R, Minniti G, Preusser M, et al. . EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 6. Zamanipoor Najafabadi AH, Peeters MCM, Dirven L, et al. . Impaired health-related quality of life in meningioma patients—a systematic review. Neuro Oncol. 2017;19(7):897–907. doi: 10.1093/neuonc/now250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zamanipoor Najafabadi AH, Peeters MCM, Lobatto DJ, et al. . Health-related quality of life of cranial WHO grade I meningioma patients: are current questionnaires relevant? Acta Neurochir (Wien). 2017;159(11):2149–2159. doi: 10.1007/s00701-017-3332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landelijke Werkgroep Neuro-oncologie. Oncoline Richtlijnen Oncologische Zorg Intracranieel Meningeoom. Integraal Kankercentrum Nederland; http://www.oncoline.nl/meningeoom. Published 2015. Accessed December 12, 2017. [Google Scholar]
- 9. Etikan I, Musa S, Alkassim R. Comparison of convenience sampling and purposive sampling. Am J Theor Appl Stat. 2016;5(1):1–4. doi: 10.11648/j.ajtas.20160501.11. [DOI] [Google Scholar]
- 10. Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201–205. doi: 10.4997/JRCPE.2015.305. [DOI] [PubMed] [Google Scholar]
- 11. Meesters J, Pont W, Beaart-Van De Voorde L, Stamm T, Vliet Vlieland T.. Do rehabilitation tools cover the perspective of patients with rheumatoid arthritis? A focus group study using the ICF as a reference. Eur J Phys Rehabil Med. 2014;50(2):171–184. [PubMed] [Google Scholar]
- 12. Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust. 2004;180(6 suppl):S57–S60. doi: 10.5694/j.1326-5377.2004.tb05948.x. [DOI] [PubMed] [Google Scholar]
- 13. Tong A, Sainsbury P, Craig J. Consolidated Criteria for Reporting Qualitative Research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 14. Andela CD, Repping-Wuts H, Stikkelbroeck NMML, et al. . Enhanced self-efficacy after a self-management programme in pituitary disease: a randomized controlled trial. Eur J Endocrinol. 2017;177(1):59–72. doi: 10.1530/EJE-16-1015. [DOI] [PubMed] [Google Scholar]
- 15. Andela CD, Scharloo M, Ramondt S, et al. . The development and validation of the Leiden Bother and Needs Questionnaire for patients with pituitary disease: the LBNQ-Pituitary. Pituitary. 2016;19(3):293–302. doi: 10.1007/s11102-016-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lobatto DJ, van den Hout WB, Zamanipoor Najafabadi AH, et al. . Healthcare utilization and costs among patients with non-functioning pituitary adenomas. Endocrine. 2019;64(2):330–340. doi: 10.1007/s12020-019-01847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lobatto DJ, Steffens ANV, Zamanipoor Najafabadi AH, et al. . Work disability and its determinants in patients with pituitary tumor-related disease. Pituitary. 2018;21(6):593–604. doi: 10.1007/s11102-018-0913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majumdar SR, Lier DA, Beaupre LA, et al. . Osteoporosis case manager for patients with hip fractures: results of a cost-effectiveness analysis conducted alongside a randomized trial. Arch Intern Med. 2009;169(1):25–31. doi: 10.1001/archinte.169.1.25. [DOI] [PubMed] [Google Scholar]
- 19. Casanueva FF, Barkan AL, Buchfelder M, et al. ; Pituitary Society, Expert Group on Pituitary Tumors Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society statement. Pituitary. 2017;20(5):489–498. doi: 10.1007/s11102-017-0838-2 [Erratum in Pituitary. 2018;21(6): 663. doi:.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jordan JT, Sanders AE, Armstrong T, et al. . Quality improvement in neurology: Neuro-Oncology Quality Measurement Set. Neuro Oncol. 2018;20(4):531–537. doi: 10.1093/neuonc/nox245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Majumdar SR, Beaupre LA, Harley CH, et al. . Use of a case manager to improve osteoporosis treatment after hip fracture: results of a randomized controlled trial. Arch Intern Med. 2007;167(19):2110–2115. doi: 10.1001/archinte.167.19.2110. [DOI] [PubMed] [Google Scholar]
- 22. Gary TL, Batts-Turner M, Yeh HC, et al. . The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2009;169(19):1788–1794. doi: 10.1001/archinternmed.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutherland D, Hayter M. Structured review: evaluating the effectiveness of nurse case managers in improving health outcomes in three major chronic diseases. J Clin Nurs. 2009;18(21):2978–2992. doi: 10.1111/j.1365-2702.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 24. Gehring K, Sitskoorn MM, Gundy CM, et al. . Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. doi: 10.1200/JCO.2008.20.5765. [DOI] [PubMed] [Google Scholar]
- 25. Formica V, Del Monte G, Giacchetti I, et al. . Rehabilitation in neuro-oncology: a meta-analysis of published data and a mono-institutional experience. Integr Cancer Ther. 2011;10(2):119–126. doi: 10.1177/1534735410392575. [DOI] [PubMed] [Google Scholar]
- 26. Bartolo M, Zucchella C, Pace A, et al. . Early rehabilitation after surgery improves functional outcome in inpatients with brain tumours. J Neurooncol. 2012;107(3):537–544. doi: 10.1007/s11060-011-0772-5. [DOI] [PubMed] [Google Scholar]
- 27. Basch E, Deal AM, Kris MG, et al. . Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moote M, Krsek C, Kleinpell R, Todd B.. Physician assistant and nurse practitioner utilization in academic medical centers. Am J Med Qual. 2011;26(6):452–460. doi: 10.1177/1062860611402984. [DOI] [PubMed] [Google Scholar]
- 29. Dirven L, Armstrong TS, Blakeley JO, et al. . Working plan for the use of patient-reported outcome measures in adults with brain tumours: a Response Assessment in Neuro-Oncology (RANO) initiative. Lancet Oncol. 2018;19(3):e173–e180. doi: 10.1016/S1470-2045(18)30004-4. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211. doi: 10.1186/1472-6963-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J.. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 32. Dawson J, Doll H, Fitzpatrick R, Jenkinson C, Carr AJ.. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186. doi: 10.1136/bmj.c186. [DOI] [PubMed] [Google Scholar]
- 33. Eysenbach G, Powell J, Englesakis M, Rizo C, Stern A.. Health related virtual communities and electronic support groups: systematic review of the effects of online peer to peer interactions. BMJ. 2004;328(7449):1166. doi: 10.1136/bmj.328.7449.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frost JH, Massagli MP. Social uses of personal health information within PatientsLikeMe, an online patient community: what can happen when patients have access to one another’s data. J Med Internet Res. 2008;10(3):e15. doi: 10.2196/jmir.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Back MF, Ang EL, Ng WH, et al. . Improvements in quality of care resulting from a formal multidisciplinary tumour clinic in the management of high-grade glioma. Ann Acad Med Singapore. 2007;36(5):347–351. [PubMed] [Google Scholar]
- 36. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 37. Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE.. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290–298. doi: 10.1136/bmjqs-2013-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sokovic M, Pavletic D, Kern Pipan K. Quality improvement methodologies—PDCA cycle, RADAR matrix, DMAIC and DFSS. J Achiev Mater Manuf Eng. 2010;43(1):476–483. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.