Abstract
Background
Studies summarizing the clinical picture of COVID-19 in children are lacking. This review characterizes clinical symptoms, laboratory, and imaging findings, as well as therapies provided to confirmed pediatric cases of COVID-19.
Methods
Adhering to PRISMA guidelines, we searched four medical databases (PubMed, LitCovid, Scopus, WHO COVID-19 database) between December 1, 2019 to May 14, 2020 using the keywords “novel coronavirus”, “COVID-19” or “SARS-CoV-2”. We included published or in press peer-reviewed cross-sectional, case series, and case reports providing clinical signs, imaging findings, and/or laboratory results of pediatric patients who were positive for COVID-19. Risk of bias was appraised through the quality assessment tool published by the National Institutes of Health. PROSPERO registration # CRD42020182261.
Findings
We identified 131 studies across 26 countries comprising 7780 pediatric patients. Although fever (59·1%) and cough (55·9%) were the most frequent symptoms 19·3% of children were asymptomatic. Patchy lesions (21·0%) and ground-glass opacities (32·9%) depicted lung radiograph and computed tomography findings, respectively. Immunocompromised children or those with respiratory/cardiac disease comprised the largest subset of COVID-19 children with underlying medical conditions (152 of 233 individuals). Coinfections were observed in 5.6% of children and abnormal laboratory markers included serum D-dimer, procalcitonin, creatine kinase, and interleukin-6. Seven deaths were reported (0·09%) and 11 children (0·14%) met inclusion for multisystem inflammatory syndrome in children.
Interpretation
This review provides evidence that children diagnosed with COVID-19 have an overall excellent prognosis. Future longitudinal studies are needed to confirm our findings and better understand which patients are at increased risk for developing severe inflammation and multiorgan failure.
Funding
Parker B. Francis and pilot grant from 2R25-HL126140. Funding agencies had no involvement in the study.
Introduction
In December 2019, an unprecedented number of pneumonia cases presented in adult individuals from Wuhan, China [1]. Despite rapid action by the Chinese government and health officials, the number of similar presenting cases continued to rise at an alarming rate [2]. By January 2020 an emerging zoonotic agent, known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), was identified in respiratory samples in patients diagnosed with pneumonia who subsequently developed respiratory failure [1]. The spread of SARS-CoV-2 from human to human, through respiratory droplets, has now resulted in a worldwide outbreak, now classified as a pandemic by the World Health Organization [3].
As of June 3rd, 2020, there has been more than 6·4 million confirmed cases worldwide and >380,000 fatalities [4] Most symptomatic cases have occurred in the adult population, characterized by fever, cough, malaise, and frequent hospitalization [1]. Accordingly, most of the published data is derived from adults with coronavirus disease 2019 (COVID-19) who were hospitalized in China [5]. As the pandemic continues, we are now observing numerous reports describing the clinical presentation and hospital course of children with confirmed COVID-19 [5].
What is currently known is that children have milder symptoms and are less likely to be hospitalized when compared to adults [6]. However, on May 14th, 2020 the United States Centers for Disease Control and Prevention (CDC) released a health advisory reporting a multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 [7]. This statement stemmed from a subset of pediatric patients manifesting with severe inflammation, multi-organ failure, and testing positive for SARS-CoV-2 [8,9].
Our goal was to conduct a systematic review: (i) to understand the clinical picture and presentation of pediatric patients with confirmed COVID-19, and (ii) to provide an initial observation of the signs, symptoms, and laboratory findings of pediatric patients who developed MIS-C.
Methods
2.1 Search strategy and selection criteria
Our methods adhere to the guidelines established by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Our study protocol was registered with PROSPERO (International Prospective Register of Systematic Reviews) under the following identifier # CRD42020182261.
We conducted a systematic search in the following databases: PubMed, LitCovid, Scopus, and the WHO COVID-19 database. Additionally, we searched for studies that included the following terms- “novel coronavirus, COVID-19, 2019-nCOV, SARS-CoV-2, pediatric, child, and neonate” into the freely accessible research domains of JAMA, Lancet, NEJM, CHEST, and Google Scholar. The last search was performed on May 14th, 2020 and was not limited by language (translation performed with Google Translate).
We included published or in press peer-reviewed articles reporting pediatric cases of confirmed COVID-19. We accepted the following types of studies: cross-sectional, cases series, case-control, case reports, review articles, opinion papers, and letters to journal editors that incorporated clinical, laboratory, imaging, and hospital course of pediatric patients. The pediatric population included neonates, children, and young adults up to 21 years of age. We set the upper limit of age to 21 years as several countries use this number to stratify their pediatric versus adult data. Patients were included if SARS-CoV-2 was detected by real time reverse transcription polymerase chain reaction (RT-PCR) in nasopharyngeal, throat, blood, or stool samples at any point of their clinical evaluation. Suspected cases of COVID-19 without positive RT-PCR were excluded in this study. Furthermore, we also excluded in vitro studies or manuscripts focusing on animal experiments.
Screening by title and abstract was conducted independently by at least two investigators (AH, KC, or AxM). A third investigator (AM) was consulted to resolve differences of opinion in either phase. Subsequent full-text review and data extraction was conducted by all investigators using a standardized online form shared among the authors. Data retrieved from each article was cross-checked by at least two independent investigators.
Our outcomes of interest were to describe the clinical signs, imaging findings, and laboratory results characteristic of pediatric patients with confirmed COVID-19. Also, we wanted to provide an initial description of children with confirmed diagnosis of SARS-CoV-2 who develop MIS-C. We used the definition by the CDC to define MIS-C
(e.g., fever, laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalization, with multisystem (≥2) organ involvement with no alternative diagnosis, and positive for SARS-CoV-2 infection) [7]. Control cases were patients from the same case series who did not meet criteria for MIS-C or studies that presented individual patient data where MIS-C could be definitively ruled out.
2.2 Data collection and risk of bias assessment
Data extraction was performed by all investigators and compared by at least two investigators for consistency. Data collected included the type of article (e.g., case series), country of origin, number of pediatric patients, demographic information, and all clinical symptoms (e.g., fever, cough), laboratory values (e.g., CBC, LFTs, BMP), imaging studies (e.g., chest x-ray, CT, MRI), clinical outcomes (e.g., ICU admission), and treatments provided (e.g. antivirals).
The risk of bias for observational studies was appraised through the quality assessment tool published by the National Institutes of Health [10]. We opted to use this guide as the development of the assessment tool was conducted rigorously by researchers in the Agency for Healthcare Research and Quality Evidence-Based Practice Centers, the Cochrane Collaboration, the United States Preventive Services Task Force, the Scottish Intercollegiate Guidelines Network, the National Health Service Centre for Reviews and Disseminations, and consulting epidemiologists. Moreover, it was a preferred tool in a systematic review on risk of bias assessments used in PROSPERO-registered protocols [11]. Risk of bias was assessed independently by at least two investigators and disagreements were resolved by a third researcher (AM). Furthermore, the level of evidence was assessed according to Sackett [12].
2.3 Data analysis
All laboratory data were converted to similar units and presented as mean with standard deviation (SD). Laboratory information presented as median (IQR) were converted to mean (SD), and denoted when unable to convert [13]. Publications that provided multiple timepoints (e.g., hospital course of individuals) for laboratory results were gathered and averaged. If the symptom was present anytime during the hospitalization, it was considered positive and characterized as a count with percent. A similar approach was taken for imaging information. Means, standard deviations, and proportion ratios were calculated using Microsoft Excel.
Statistical analyses between COVID-19 pediatric patients with/without MIS-C was conducted on STATA v·13. All statistical tests were two-sided, and significance was defined as a p value <0·05. Continuous data was summarized as mean (standard deviation) or median (interquartile range) and assessed by Student's t-test or Wilcoxon rank sum. Categorical data was summarized as counts (percent) and analyzed by Fisher's exact test.
2.4 Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
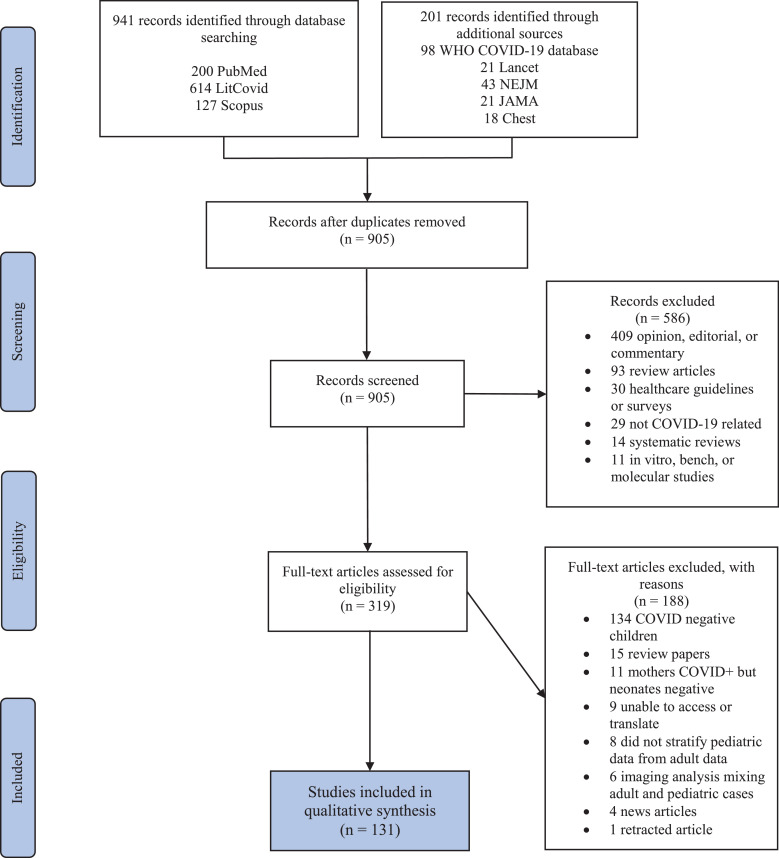
The search yielded 1,142 studies. After removing 237 duplicates, 905 articles were reviewed by abstract and title. After initial screening, only 319 articles met inclusion criteria and underwent full text evaluation. Publications that were retracted, or consisted of editorials, reviews, or commentaries that did not meet our criteria were removed, generating a final list of 131 articles (see Fig. 1).
Fig. 1.
PRISMA flow diagram.
Studies included in this review were published between January 24th to May 11th, 2020. Eight studies were cross sectional, 75 were case series, and 48 were case reports (refer to Table 1). Twenty-six countries were represented with the largest data derived from 2572 children from the United States. China comprised 64·1% of the studies included in this review. Appendix 1 displays publications by the country of origin.
Table 1.
Study characteristics.
| # | First author | Study type | Country | N | Age (years) | Male N | Clinical symptoms | Laboratory findings | Imaging characteristics | Therapy | ICU (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Aghdam, M | Case report | Iran | 1 | 0.042 | 1 (100%) | Fever, lethargy, mottling, respiratory distress | Normal CBC, BUN, Cr, and ABG | Normal CXR, no lung CT | Fluids, oxygen, antibiotics, oseltamivir | 1 |
| 2 | Almeida, F | Case report | Brazil | 1 | 10 | 0 (0%) |
Fever, cough, sore throat, gross hematuria | Urinalysis showed normal shaped red cells | NR | NR | 0 |
| 3 | Alonso Diaz, C | Case report | Spain | 1 | 0.022 | 0 (0%) |
Tachypnea, retractions, and desaturations 9 days after birth | Normal CRP and capillary gas | CXR: ground glass opacities in the right perihilar region | Observation | 1 |
| 4 | An, P | Case report | China | 1 | 3 | 0 (0%) |
Asymptomatic | NR | CT: bilateral consolidation and ground-glass opacities | Antibiotic | 0 |
| 5 | Andina, D | Case series* | Spain | 1 | NR | 0 (0%) |
Mild gastrointestinal symptoms and chilblains on feet | NR | NR | Oral analgesic, antihistamine, topical corticosteroids for some patients | NR |
| 6 | Andre, N | Case series | France | 5 | 9.6 | 2 (40%) |
Respiratory compromise in children with oncologic disease | NR | NR | NR | 5 |
| 7 | Bi, Q | Case series | China | 32 | NR | NR | Time to recovery better in children <9 years of age (17.5 days) vs. 10-19 years (19.1 days), secondary household attack rate ~7.25% | NR | NR | NR | NR |
| 8 | Cai, J | Case series | China | 10 | 6.17 | 4 (40%) |
7 patients with fever, 6 with cough, 4 with sore throat, 0 with diarrhea, all patients were symptomatic | ↑CRP (n=8), ↑PCT (n=6), ↑LDH (n=3); ↑WBC (n=3), ↑D-dimer (n=2) | CXR: unilateral patchy infiltrates (n=4) | Symptomatic treatment (n=5), symptomatic treatment + antibiotics (n=5) | 0 |
| 9 | Cai, JH | Case report | China | 1 | 7 | 1 (100%) |
Fever, cough, rhinorrhea, nausea | ↑WBC, CRP, and D-dimer | CXR: Bilateral thickening of lung texture | Observation, Chinese medications | 0 |
| 10 | Calvo, C | Cross sectional | Spain | 5 | 2.5 | 0 (0%) |
NR | NR | NR | NR | NR |
| 11 | Canarutto, D | Case report | Italy | 1 | 0.088 | 1 (100%) |
Fever, cough, and rhinorrhea | Mild neutropenia, monocytosis, and reactive lymphocytes on blood smear | CXR:normal | NR | NR |
| 12 | Carrabba, G | Case report | Italy | 1 | 0.67 | 1 (100%) |
Mild temperature, dry cough | NR | CXR:no overt interstitial pneumonia but mild veiling opacity of left lung, no lung CT | Neurosurgery for shunt revision x2 | 0 |
| 13 | CDC COVID 19 Response team | Case series | USA | 2572 | 9.8 | 1408 (57%) | Symptom data available for 291 patients: 56% of pediatric patients reported fever, 54% reported cough, and 13% reported shortness of breath, 53 of 78 cases did not report symptoms, 23% (n=80) of 345 patients had at least 1 underlying medical condition, 3 deaths | NR | NR | NR | 15 |
| 14 | Cela, E | Case series | Spain | 15 | 10.1 | 14 (93%) |
Fever (n=10), cough (n=6), asymptomatic (n=2), hypoxemia (n=2), all patients with oncologic disease | median WBC 3.2, median lymphocyte 18.2%, median D-dimer normal | CXR: normal (n=6), pneumonia (n=4), peribronchial cuffing (n=4) | Hydroxychloroquine (n=11), tocilizumab & lopinavir/ritonavir (n=1), oxygen (n=2), antibiotic (n=2), remdesivir (n=1), no treatment (n=4) | NR |
| 15 | Chacon-Aguilar, R | Case report | Spain | 1 | 0.07 | 1 (100%) |
Paroxysmal episodes with generalized hypertonia, fever, rhinorrhea, vomiting, diarrhea | CBC, liver and kidney studies normal, ↑CK (380 U/L), ↑LDH (390 U/L), normal CRP | NR | Antibiotics | 0 |
| 16 | Chan, JF | Case series* | China | 1 | 10 | 1 (100%) |
Asymptomatic | Normal CBC, fibrinogen, CRP, Cr, LDH, and CK, ↑alkaline phosphatase | CT: bilateral ground-glass opacities | NR | 0 |
| 17 | Chang, D | Case series | China | 2 | 8.5 | NR | Fever, cough | NR | NR | NR | 0 |
| 18 | Chen, F | Case report | China | 1 | 1.08 | 1 (100%) |
Fever, shortness of breath, vomiting, diarrhea, myalgia/fatigue, cold limbs with poor perfusion | Normal ABG, coagulation profile; LFTs, ↑BUN, Cr, CK, serum amyloid, IL-6, IL-10 | CXR: large blurred image of the upper and lower right lung; CT: enhanced texture of both lungs, large consolidation on the right, ground-glass shadow | Continuous dopamine, IV bolus, ventilator assistance, correction of acidosis, interferon, glucocorticoid, oseltamivir, antibiotics, abdominal decompression | 1 |
| 19 | Chen, H | Case report | China | 1 | 12 | 1 (100%) |
Fever, cough, abdominal pain, sputum production, no vomiting | Normal CBC, ↑CRP | CT: pneumonia in the right upper lung, followed by bilateral ground-glass opacities | Antibiotics, arbidol, and supplemental oxygen | NR |
| 20 | Chen, J | Case series | China | 12 | 14.5 | 6 (50%) |
Cough (75%), fever (58.3%), diarrhea (33%), dizziness (16.7%), sore throat (16.7%) | Normal CBC, LFTs, BUN, PT, ↑total B and T cells when compared to adults, but comparable NK cell, IgM, IgG, and C3 | Ground-glass opacity was the most common finding on chest CT | All patients received interferon, 8 received lopinavir/ritonavir, and 2 received ribavirin | 0 |
| 21 | Cui, Y | Case report | China | 1 | 0.15 | 0 (0%) |
Pharyngeal hyperemia, rhinorrhea, cough, sputum | Slightly elevated IgM, lymphocyte, and platelet counts; normal Hgb, D-dimer, PTT, PT, CRP, ESR, and renal function | CT: Unilateral ground-glass opacity in the right lung and unilateral consolidation | Interferon, antibiotic, ursodeoxycholic acid, Chinese medicine | 1 |
| 22 | de Rojas, T | Case series | Spain | 15 | 10.1 | 14 (93%) |
10 patients had fever, 6 patients had cough, 1 with hypoxemia, and 2 asymptomatic patients; all patients had an underlying oncologic disease | Median WBC count was 3,195 and median lymphocyte count was 580 | CXR: normal (n=6), pneumonia (n=4), peribronchial cuffing (n=4) | 11 patients received hydroxychloroquine and 2 received antibiotics, tocilizumab (n=1), lopinavir-ritonavir (n=1), glucocorticoid (n=1), and remdesivir (n=1) | 0 |
| 23 | Denina, M | Case series | Italy | 8 | 4.2 | 5 (63%) |
Fever (n=6), dry cough (n=5), dyspnea (n=3), pharyngeal congestion (n=3), vomiting or diarrhea (n=3), hypoxemia (n=2) | NR | CXR: pulmonary consolidation (n=1), ground-glass opacities (n=4); LUS: confluent B-lines (n=5), subpleural consolidations (n=2) | Oxygen (n=2) | NR |
| 24 | Dona, D | Case series | Italy | 2 | 0.29 | NR | Fever (n=1) diarrhea (n=1), respiratory symptoms in both | NR | NR | NR | 0 |
| 25 | Dong, L | Case report | China | 1 | 0.003 | 0 (0%) |
Asymptomatic | Nasopharyngeal test was negative, but IgM and IgG were elevated 2 hours after birth, ↑IL-6, IL-10, LDH | CT: normal | NR | 1 |
| 26 | Dong, Y | Case series | China | 731 | 10 | 420 (58%) |
315 mild illness, 300 moderate illness, 18 severe and 3 critically ill, 94 asymptomatic children | NR | NR | NR | NR |
| 27 | Du, W | Case series | China | 14 | 7.1 | 6 (43%) |
Fever (n=5) and cough (n=3) were commonly reported; Eight (57.1%) were asymptomatic | ↑LDH (n=7), ↑PCT (n=5), ↑D-dimer (n=5), ↑CK (n=4), leukopenia (n=4), ↑IL-6 (n=1) | CT: bilateral lung injury (n=6) and unilateral (n=5) | NR | 0 |
| 28 | Fan, Q | Case report | China | 1 | 0.25 | 0 (0%) |
Fever and diarrhea | Neutrophilia (86.2%), lymphopenia (7.1%) | CT: normal | Supportive care | 0 |
| 29 | Feng, K | Case series | China | 15 | 7 | 4 (33%) |
Asymptomatic (n=8), fever (n=5), cough or nasal congestion (n=1) | ↓WBC (n=8), normal WBC (n=7) | CT: ground glass lesions (n=7), no lesions (n=6), patchy shadow (n=2) | NR | NR |
| 30 | Ferrazzi, E | Case series | Italy | 3 | 0.003 | NR | 1 neonate with gastrointestinal and respiratory symptoms 3 days after birth | NR | NR | NR | 1 |
| 31 | Genovese, G | Case report | Italy | 1 | 8 | 0 (0%) |
Fever, cough, papulovesicular rash to trunk | Normal complete blood count, CRP, liver and kidney function, mild thrombocytopenia (105k) | NR | NR | NR |
| 32 | Guan, W | Case series | China | 9 | NR | NR | NR | NR | NR | NR | NR |
| 33 | Gubjartsson, D | Cross sectional | Iceland | 1321 | NR | NR | NR | NR | NR | NR | NR |
| 34 | Han, M | Case report | Korea | 1 | 0.07 | 0 (0%) |
Fever, cough, and vomiting; viral shedding in urine and stool for 10 and 18 days, respectively | First CBC with mild neutropenia (817 per mm3) | CXR: normal | No antibiotics or antivirals | 0 |
| 35 | Han, Y | Case series | China | 7 | 4 | 4 (57%) |
Fever (n=5), cough (n=5), shortness of breath (n=3), vomiting (n=4), diarrhea (n=4), sore throat (n=1), myalgia (n=1) | ↑BNP (n=5), ↑CK (n=4), ↑PCT (n=3), ↑AST (n=3), ↑LDH (n=2), ↑CRP (n=2) | Pneumonia on CT and CXR (n=5) | Oxygen therapy (n=2) glucocorticoids (n=1) | 0 |
| 36 | Hrusak, O | Cross sectional | Czech Republic, USA, Italy, Spain, Switzerland, Denmark, Austria, Sweden, Belgium, Netherlands | 9 | NR | NR | Fever (n=7) and diarrhea (n=1) were the most common symptoms in this cohort of children with oncologic disease | Lymphopenia (n = 1), neutropenia (n=5) | Normal CXR in 1 patient, all others NR | Antibiotics (n=2) lopinavir/ritonavir (n=1) hydroxychloroquine (n=2) | 0 |
| 37 | Hu, X | Case series* | China | 1 | 0.004 | 1 (100%) |
Asymptomatic | WBC, Hgb, Plts, CRP, Cr, ALT normal, ↑PCT (n=6) | Normal CXR=1; no CT data | NR | 0 |
| 38 | Ibrahim, L | Case series | Australia | 4 | 13.1 | 1 (25%) |
Sore throat (n=4), headache/dizziness (n=3), cough (n=2), fever (n=1) | NR | NR | None | 0 |
| 39 | Ji, L | Case series | China | 2 | 12 | 2 (100%) |
Fever and diarrhea (n=1) | ↑WBC (n=1), ↑CRP (n=1) | CT: normal (n=2) | Symptomatic treatment, oral probiotic | 0 |
| 40 | Ji, T | Case series | China | 19 | NR | NR | Asymptomatic (n=9) | NR | NR | NR | NR |
| 41 | Jiang, S | Case series | China | 2 | 5.08 | 0 (0%) |
Fever, cough, and vomiting in both patients | ↑WBC, neutrophil count, CRP, PCT, serum amyloid A (n=1) | CT: normal (n=1), bilateral ground-glass opacities with patchy shadows (n=1) | Antibiotics (n=2), oseltamivir (n=1), glucocorticoids (n=1), IVIG (n=1) | 1 |
| 42 | Jones, V | Case report | USA | 1 | 0.5 | 0 (0%) |
Fussy, conjunctivitis, dry cracked lips, prominent tongue papilla, polymorphous maculopapular rash, swelling of hands and feet, fever, anorexia | WBC with bandemia, ↑CRP, normal ESR, BMP, and LFTs | CXR: faint opacity in left midlung | IVIG and acetylsalicylic acid | NR |
| 43 | Kam, K | Case report | Singapore | 1 | 0.5 | 1 (100%) |
Asymptomatic initially, followed by fever | Viremia, normal CBC, LFTs | NR | NR | NR |
| 44 | Kan, M | Case report | USA | 1 | 0.02 | 0 (0%) |
Fever, tachycardia, cough; underlying condition of hydronephrosis and duplicating renal system | Leukopenia, lymphopenia, neutropenia, normocytic anemia, normal platelets, normal CRP | NR | Bolus, antibiotic, antipyretics | 0 |
| 45 | Korean Society of Infectious Diseases | Cross sectional | Korea | 201 | NR | NR | NR | NR | NR | NR | NR |
| 46 | Lai, W | Case series | China | 2 | 14 | 2 (100%) |
2 with dry cough, 2 with fever, and 1 with malaise | NR | CT: unilateral patchy ground-glass opacities (n=2) | Antivirals, supportive | 0 |
| 47 | Le, H | Case report | Vietnam | 1 | 0.25 | 0 (0%) |
Rhinorrhea and nasal congestion, fussy | Normal CBC, CK, LDH, CRP, and PCT | CXR:normal | Antibiotic | NR |
| 48 | Leva, E | Case series | Italy | 16 | NR | NR | All patients with fever and cough | NR | NR | NR | 4 |
| 49 | Li, H | Case series | China | 40 | 5.1 | 23 (58%) |
Cough (n=27), fever (n=21), myalgia (n=4), diarrhea (n=4), rhinorrhea (n=2), sore throat (n=2) | Normal CBC, CRP, PCT, ↑CD3+, CD8+ lymphocyte (n=40), ↑% of CD3+ (n=40), and ↓ percentage of CD19+ lymphocyte (n=40) | CT: unilateral (n=13), bilateral (n=26) ground-glass opacities, normal (n=1) | Interferon (n=40), oseltamivir (n=20), IVIG (n=4), steroids (n=3), azithromycin (n=13), mechanical ventilation (n=1) | 1 |
| 50 | Li, J | Case report | China | 1 | 0.67 | 0 (0%) |
Cough | WBC and differential, PT, D-dimer, LFTs and renal function normal, ↑CRP | Normal CT | Interferon | 0 |
| 51 | Li, M | Case report | China | 1 | 0.006 | 1 (100%) |
Patient had no fever or cough | NR | NR | NR | 0 |
| 52 | Li, W | Case series | China | 4 | 7.2 | 4 (100%) |
Cough | NR | NR | NR | NR |
| 53 | Li, Wei | Case series | China | 5 | 3.4 | 4 (80%) |
Asymptomatic (n=4), 1 patient with rhinorrhea, cough, sore throat, and fever | ↑WBC (n=2), ↑CRP (n=1) | CT: patchy ground-glass opacities (n=3), normal (n=2) | IVIG (n=5), antivirals (n=2), montelukast (n=3), interferon (n=2) | NR |
| 54 | Li, Y | Case series | China | 2 | 4 | 1 (50%) |
Cough and rhinorrhea (n=1) | ↓neutrophils (n=1), ↑CRP (n=1), normal coagulation profile, LFTs and renal function in both patients | CT: bilateral spots upper lobes (n=1), increased bronchovascular bundles bilaterally (n=1) | Oxygen (n=1) | 0 |
| 55 | Lin, J | Case report | China | 1 | 7 | 0 (0%) |
Nasal congestion and dry cough; no fever, dyspnea, or diarrhea | NR | CT showed no signs of pneumonia | Supportive treatment, oseltamivir, and interferon | NR |
| 56 | Liu, M | Case series | China | 5 | 6.4 | 4 (80%) |
3 were asymptomatic, 2 patients with fever and dry cough | Normal WBC, mild neutropenia (n=3) | CT: unilateral (n=3) and bilateral (n=1) ground-glass opacities | Interferon (n=4), ribavirin (n=3) | NR |
| 57 | Liu, W | Case series | China | 6 | 3.5 | 2 (33%) |
High fever and cough in all patients; vomiting (n=4) | White cells (n=4), lymphocytes (n=6), and neutrophils (n=3) were decreased | CT: patchy shadows bilaterally (n=3), patchy ground-glass opacities in both lungs (n=1), normal (n=1) | Oseltamivir (n=6), glucocorticoid (n=4), ribavirin (n=2), IVIG (n=1) | 1 |
| 58 | Liu, Y | Case report | China | 1 | 10 | 1 (100%) |
Asymptomatic | Normal CBC, LFTs, ↑LDH, borderline ↑CRP | CT: ground-glass opacity and pleural effusion | Ribavirin, interferon | 0 |
| 59 | Locatelli, A | Case report | Italy | 1 | 16 | 1 (100%) |
Mild diarrhea and chilblain-like lesions to fingers and a toe | Coagulation, autoimmunity, cryoglobulins normal | NR | NR | NR |
| 60 | Lou, X | Case series | China | 3 | 4.8 | 1 (33%) |
All patients had fever; 2 with fatigue, nasal congestion, diarrhea, and headache | NR | NR | Interferon (n=2) | 0 |
| 61 | Lu, X | Case series | China | 171 | 6.4 | 104 (61%) |
Fever (41.5%), pharyngeal erythema (46.2%), diarrhea (8.8%), asymptomatic (15.8%), 1 death | ↓WBC (26.3%), lymphopenia (3.5%), ↑PCT (64%), ↑CRP (19.7%), ↑D-dimer (14.1%), ↑AST (14.6%) | CT: ground glass opacity (32.7%), unilateral or bilateral patchy shadowing (31%), interstitial abnormalities (1.2%) | NR | 3 |
| 62 | Lu, Y | Case series | China | 9 | 7.8 | 5 (56%) |
Fever (n=6), cough (n=3), asymptomatic (n=1) | All WBC counts were normal | CXR: no overt abnormality (n=5); CT: no overt abnormality (n=4), patch ground-glass opacities (n=4) | NR | 0 |
| 63 | Lu, Yingying | Case series | China | 110 | 5.8 | 59 (53%) |
Cough and dyspnea (51.8%), followed by fever (50.9%) were the most common symptoms, 26 (23.6%) patients had gastrointestinal symptoms, 29 (26.4%) were asymptomatic | Symptomatic patients were more likely to have a ↓Hgb (16.4% vs. 0%), ↑AST (23.5% vs. 0%), and trended towards an ↑IL-6 (12% vs. 0%) | 64 patients had a chest x-ray demonstrating pneumonia | All received antivirals, interferon was the most frequently used, Chinese medication (n=22) | 0 |
| 64 | Ma, H | Case series | China | 50 | 3.3 | 28 (56%) |
32 with fever, 22 with cough, 8 with rhinorrhea, 1 with sore throat, 2 with myalgia, 3 with diarrhea, 6 with no symptoms | Leukocytosis (n=2), leukopenia (n=19), polycythemia (n=2), thrombocytopenia (n=7), ↑CRP (n=10) | CT: ground-glass opacities (n=29), local patchy shadowing (n=9), normal (n=7) | NR | NR |
| 65 | Ma, H2 | Case series | China | 22 | 5.5 | 12 (55%) |
Fever (n=13), dry cough (n=5), shortness of breath (n=1), asymptomatic (n=2) | NR | CT: ground-glass shadows (n=6), consolidation (n=4), consolidation and ground-glass shadows (n=6), bronchial pneumonia-like changes (n=3), normal (n=3) | NR | NR |
| 66 | Ma, Y | Case series | China | 115 | NR | 73 (64%) |
Asymptomatic (n=61), fever (n=29), cough (n=47), rhinorrhea (n=47), gastrointestinal symptoms (n=3) | Normal WBC (n=88), ↓WBC (n=23); lymphocytes normal (n=60), ↑lymphocytes (n=40); normal neutrophils (n=77),↑neutrophils (n=32), ↑ALT (n=11), ↑CK-MB (n=34), ↑BUN/Cr (n=2) | CT: ground-glass opacities (n=49), normal (n=27) | NR | NR |
| 67 | Mansour, A | Case report | Lebanon | 1 | 1.33 | 0 (0%) |
Patient presented with fever, diarrhea, and decreased activity | Leukocytosis, elevated platelets, elevated CRP, decreased hemoglobin/hematocrit | CXR: unilateral, large consolidation with bronchial infiltrate | Hydration and antibiotics | 0 |
| 68 | Mao, L | Case report | China | 1 | 1.16 | 1 (100%) |
Patient presented with fever, cough, congestion, rhinorrhea, decreased appetite | Normal CBC, PCT, LFTs, renal function, D-dimer; normal T cell, B cell, and NK cell, ↑CRP | CT: unilateral ground-glass opacities in right lower lung | Interferon and supportive | 0 |
| 69 | Mizumoto, K | Case series | Japan | 3 | NR | NR | Asymptomatic (n=2) | NR | NR | NR | NR |
| 70 | Morey-Olive, M | Case series | Spain | 2 | 3 | 1 (50%) |
Low grade fever (n=2) | Abnormal liver enzymes and coagulation parameters in 1 patient | NR | NR | 0 |
| 71 | Munoz, A | Case report | USA | 1 | 0.06 | 1 (100%) |
Nasal congestion, tachypnea, reduced feeding, subsequent pneumothorax | ↑PCT (6.53 ng/mL) and ↑CRP (172 mg/L) | CXR: bilateral linear opacities and consolidation of right upper lobe | Mechanical ventilation, antibiotics, hydroxychloroquine, vasopressors | 1 |
| 72 | Nathan, N | Case series | France | 5 | 0.18 | 5 (100%) |
All had fever, 4 patients with hypotonia or drowsiness and moaning, 4 with cough and rhinorrhea | CBC normal, ↑CRP (n=3), ↑PCT (n=1) | Normal CXR in 4 patients, 1 patient with hyperinflation | Antipyretics | 0 |
| 73 | Ng, K | Case series | UK | 8 | 0.39 | 2 (25%) |
Fever (n=5), anorexia (n=4), tachypnea (n=2), skin mottling (n=1) | 2 patients had neutropenia and thrombocytosis | 2 patients had some opacities on CXR | 4 patients treated with broad-spectrum antibiotics | 2 |
| 74 | Odievre, M | Case report | France | 1 | 16 | 0 (0%) |
Fever, followed by acute chest syndrome in a patient with sickle cell disease | ↑CRP, ↑D-dimer, ↑IL-6, ↑LDH, ↑TNF-α | CT: bilateral pulmonary embolisms and bilateral consolidation with halo sign on right | Acetaminophen, non-invasive ventilation, blood transfusion, anticoagulation, tocilizumab | 1 |
| 75 | Pan, A | Case series | China | 536 | NR | NR | NR | NR | NR | NR | NR |
| 76 | Park, J | Case report | Korea | 1 | 10 | 0 (0%) |
Low-grade fever and sputum production | CBC, CRP normal, stool sample remained positive for 17 days after symptom onset | CT: unilateral patchy or nodular consolidations with peripheral ground-glass opacities | None | 0 |
| 77 | Parri, N | Case series | Italy | 100 | 6 | 57 (57%) |
Fever (n=54), cough (n=44), rhinorrhea (n=22), asymptomatic (n=21), shortness of breath (n=11), nausea (n=10), vomiting (n=10), diarrhea (n=9), myalgia (n=9), sore throat (n=4), headache (n=4); 27 patients with underlying medical conditions | WBC normal (n=40), ↓WBC (n=11), lymphocytopenia (n=14), ↑PCT (n=4), ↑LDH (n=22), ↑ALT (n=8), ↑AST (n=10) | CXR: interstitial abnormality (n=14), normal (n=15), consolidation (n=6), pleural effusion (n=1); LUS: interstitial syndrome (n=9), small sub-pleural consolidations (n=4) | Non-invasive ventilation; mechanical ventilation (n=1) | 9 |
| 78 | Patek, P | Case report | USA | 1 | 0.04 | 1 (100%) |
Fever, hypoxemia | Normal CBC, mild elevation to AST and ALT, CSF unremarkable | CXR: bilateral perihilar streaking without focal consolidation | Oxygen, empiric antibiotics, acyclovir | 1 |
| 79 | Patel, P | Case report | USA | 1 | 12 | 0 (0%) |
Fever, cough, vomiting, hematuria, and respiratory failure | Severe thrombocytopenia (<10k per μL), elevated inflammatory markers (CRP, PCT, ferritin) | CXR: bilateral diffuse airspace opacities and small pleural effusion |
IVIG, corticosteroids, mechanical ventilation, nitric oxide, azithromycin,hydroxychloroquine, tocilizumab | 1 |
| 80 | Piersigilli, F | Case report | Belgium | 1 | 0.002 | 0 (0%) |
No COVID-related symptoms; child is an extremely premature neonate | ↓WBC and lymphopenia | Normal radiographic findings | Continuous positive airway pressure | 1 |
| 81 | Qian, G | Case series* | China | 1 | 1.08 | 0 (0%) |
Asymptomatic | NR | CT: normal | NR | 0 |
| 82 | Qiu, H | Case series | China | 36 | 8.3 | 23 (64%) |
Fever (n=13), cough (n=7), headache (n=3), vomiting/diarrhea (n=2) | Leukopenia (n=7), ↓lymphocytes (n=11), ↑PCT (n=6), ↑CK-MB (n=11) | CXR: ground-glass opacities (n=19) | Interferon (n=36), lopinavir/ritonavir (n=14), oxygen (n=6) | NR |
| 83 | Qiu, L | Case report | China | 1 | 0.66 | 1 (100%) |
Fever, cough, wheezing, apnea, mottled skin, petechiae, cold fingers; patient with cardiac history | Initial labs demonstrated lymphopenia, ↓CD3+, ↓CD4+, ↓CD8+, ↓fibrinogen, ↑LDH, normal PCT and renal function | CXR: increased density, profusion, thickened lung texture; CT: multiple ground-glass opacities and patchy, high density shadows | IVIG, lopinavir/ritonavir, methylprednisolone, fluids, electrolytes, pressors | 1 |
| 84 | Robbins, E | Case report | USA | 1 | 0.16 | 1 (100%) |
Fever | CBC within normal limits, CMP normal except for a mildly elevated alkaline phosphatase and calcium | CXR: normal | Antibiotics, supportive | NR |
| 85 | Schwierzeck, V | Case series | Germany | 3 | 10 | NR | Asymptomatic (n=2), fever (n=1), cough (n=1), nasal congestion (n=1) in patients with renal disease | NR | NR | NR | NR |
| 86 | See, K | Case series | Malaysia | 4 | 6.4 | 3 (75%) |
Mild fever and diarrhea (n=1); rhinorrhea (n=1), cough and fever (n=1 mild), asymptomatic (n=1) | NR | CXR: perihilar opacities (n=2) | Antipyretics (n=2), antibiotic (n=1), rehydration (n=1), salbutamol (n=1) | 0 |
| 87 | Shekerdemian, L | Cross sectional | USA, Canada | 48 | 11.3 | 25 (52%) |
11 patients (23%) with multi-organ failure, 73% (n=35) with pulmonary symptoms, 40% (n=19) of children were medically complex | NR | NR | No medications (n=20), hydroxychloroquine (n=21), 17% underwent antiviral therapy, tocilizumab (n=5), mechanical ventilation (n=18), azithromycin (n=8) |
|
| 88 | Shen, Q | Case series | China | 9 | 7.5 | 3 (33%) |
2 asymptomatic, 3 with fever, 1 with diarrhea, sore throat, or cough, and 1 with fever and diarrhea | ↑WBC (n=1), ↑lymphocyte count (n=1), ↑CRP (n=1), ↑ESR (n=4), ↑LDH (n=4) | Normal chest x-ray and lung CT in 7 patients, 2 (22.2%) with small ground-glass opacities | All received oxygen and lopinavir/ritonavir, antibiotic treatment for 5 children, glucocorticoids and IVIG for 1 patient | 0 |
| 89 | Shi, B | Case report | China | 1 | 0.23 | 1 (100%) |
Cough, wheeze, dyspnea | WBC normal, ↑lymphocyte and platelet count; IgG, IgM, IgA, T, B, and NK cells normal, LFTs normal, RSV+ | CT: left lower lobe consolidation | Antibiotics, CPAP, IVIG, corticosteroids, interferon, Chinese medication | 1 |
| 90 | Shi, Y | Cross sectional | China | 10 | 6 | 5 (50%) |
NR | NR | NR | NR | 0 |
| 91 | Sieni, E | Case report | Italy | 1 | 1.08 | 0 (0%) |
Fever, vomiting, and diarrhea; patient with underlying oncologic disease | Leukopenia, anemia, thrombocytopenia | CXR: bilateral reticular findings | Antifungal, antibiotics, hydroxychloroquine, lopinavir/ritonavir | 0 |
| 92 | Sinelli, MT | Case report | Italy | 1 | 0.006 | 1 (100%) |
Hypoxemia, perioral cyanosis, poor sucking | Normal complete blood count and C-reactive protein | CT: mild bilateral ground-glass opacities | Oxygen support | 1 |
| 93 | Song, R | Case series | China | 7 | 3.5 | 1 (14%) |
Most asymptomatic, only 2 had fever | Normal WBC, ↓neutrophils, ↑LDH, normal fibrinogen | NR | All patients received supportive care, interferon, lopinavir/ritonavir | NR |
| 94 | Song, W | Case series | China | 16 | 7.9 | 10 (63%) |
Asymptomatic (n=8), cough (n=6), fever (n=5) | Leukocytes normal (n=14), CRP normal (n=15), liver, renal, coagulation, electrolytes, and myocardial labs were normal, ↑LDH (n=3) | CT: normal (n=5), bilateral ground-glass opacities (n=8), bilateral consolidation (n=1), patchy/nodular shadow (n=3) | Oseltamivir (n=11), antibiotics (n=9), lopinavir/ritonavir (n=4), Chinese medicine (n=13), arbidol (n=6) | 0 |
| 95 | Su, L | Case series | China | 9 | 3.5 | 3 (33%) |
Asymptomatic (n=6), fever or cough (n=3) | ↑CK-MB (n=6), ↓WBC (n=2), LFTs normal, inflammatory markers (CRP, PCT, ESR, IL-6) were normal in all patients, stools positive in 5 children warranting readmission | CT/x-ray: normal (n=5), bronchitis (n=2), pulmonary consolidation and ground-glass opacities (n=1), bronchopneumonia (n=1) | Interferon given to all patients; ribavirin (n=1) | 0 |
| 96 | Sun, D | Case series | China | 8 | 6.6 | 6 (75%) |
Tachypnea (n=8), fever or cough (n=6 each), sputum production (n=4), nausea/vomiting (n=4), diarrhea (n=3), fatigue or headache (n=1 each) | Normal/↑ WBC (n=7), ↑CRP, ↑PCT, ↑LDH (n=6), abnormal LFTs (n=4) | CT/x-ray: multiple patch-like shadows (n=6), ground-glass opacities (n=6), unilateral pneumonia (n=2), bilateral pneumonia (n=6) | Oxygen (n=6), mechanical ventilation (n=2), all patients received antivirals (virazole, oseltamivir, interferon), antibiotics (n=5), glucocorticoids (n=5), IVIG (n=4), Chinese medications (n=4) | 3 |
| 97 | Sun, K | Case series | China | 13 | NR | NR | NR | NR | NR | NR | NR |
| 98 | Sun, M | Case series* | China | 1 | 0.02 | 1 (100%) |
NR | NR | NR | NR | 0 |
| 99 | Tagarro, A | Cross sectional | Spain | 41 | 3.3 | 18 (44%) |
Upper respiratory symptoms in 14 (34%), fever (n=11), gastroenteritis or vomiting (n=2) | NR | NR | 25 (60%) required hospitalization, 2 received noninvasive ventilation and 1 was intubated | 4 |
| 100 | Tan, X | Case series | China | 13 | 7.9 | 4 (31%) |
Respiratory symptoms (n=7), cough (n=6), low fever (n=6), sore throat (n=2), asymptomatic (n=2) | LFTs, myocardial enzymes, PCT, coagulation, ferritin were normal, ↑ESR (n=3), CRP level increased (13.2 mg/L) | CT: normal (n=7); abnormal: cord-like shadows (n=2), showed ground glass shadows (n=2), had patchy high-density shadow (n=2) |
Lopinavir/ritonavir (n=12), interferon (n=10), arbidol (n=6) | 0 |
| 101 | Tan, Y | Case series | China | 10 | 7 | 3 (30%) |
4 patients with fever, 3 with respiratory symptoms, and 1 with vomiting | Normal CBC (n=9), ↑WBC and lymphocytes (n=1), ↑AST (n=2), CRP, LDH, and ferritin normal in all patients, mycoplasma+ (n=3) | CT: ground-glass opacities (n=5) | All patients treated with symptomatic support | 0 |
| 102 | Tang, A | Case report | China | 1 | 10 | 1 (100%) |
Asymptomatic | CBC:normal | CT: normal | Arbidol, interferon, Chinese medication | 0 |
| 103 | Tong, Z | Case series* | China | 1 | 12 | 1 (100%) |
NR | NR | NR | NR | NR |
| 104 | Turner, D | Case series | Israel, China, Spain, Italy, Korea, USA, UK, Portugal, France | 8 | 16 | 5 (63%) |
Fever (n=3), cough (n=3), myalgia/fatigue (n=4) in children with inflammatory bowel disease | NR | NR | 5ASA (n=4), infliximab (n=2), thiopurines (n=4), glucocorticoids (n=1) | 0 |
| 105 | Wang, D | Case series | China | 31 | 7.1 | 15 (48%) |
Asymptomatic (n=4), fever (n=20), cough (n=14), fatigue and diarrhea (n=3 each), sore throat (n=2), headache/dizziness (n=3), rhinorrhea (n=2), vomiting (n=2) | ↓Leukocytes and lymphocytes (n=2), ↑CRP (10%), ↑PCT (4%), ↑ESR (19%), ↑transaminases (22%), renal function normal | CT lung changes in 14 children, 9 of which showed patchy ground-glass opacities | Interferon (n=10), Antibiotics (n=6), oseltamivir (n=1), 18 were a combination of interferon, oseltamivir, ribavirin, arbidol, and/or lopinavir/ritonavir |
0 |
| 106 | Wang, H | Case report | China | 1 | 8 | 1 (100%) |
Fever | NR | CT: left lower lobe ground-glass opacity | Antiviral and symptomatic treatment | NR |
| 107 | Wang, J | Case report | China | 1 | 0.05 | 1 (100%) |
Fever, cough, vomiting, diarrhea | On admission: ↓WBC, ↑monocytes, ↓Plts | CT: bilateral pneumonia and bilateral ground-glass opacities | Interferon | NR |
| 108 | Wang, S | Case report | China | 1 | 0.003 | 1 (100%) |
Asymptomatic | Lymphopenia, ↑AST, ↑CK, ↑direct and total bilirubin | CT: unilateral ground-glass opacities | Antibiotic, vitamin K, bolus | 1 |
| 109 | Wang, Y | Case series | China | 43 | 6.92 | 21 (49%) |
The most common symptoms were dyspnea (87.5%), fever (62.5%), and cough (62.5%) | IL-6, IL-10, D-dimer, total bilirubin, and uric acid were elevated in severe cases | All severe cases had lesions on chest CT; ground-glass opacities (n=24), patchy consolidation (n=9) | All severe cases received supplemental oxygen; 5 placed on non-invasive respiratory mode and 3 were intubated | NR |
| 110 | Wei, M | Case series | China | 9 | 1.1 | 2 (22%) |
Asymptomatic (n=6), fever (n=4), cough (n=2), rhinorrhea (n=1), sputum production (n=1) | NR | NR | NR | 0 |
| 111 | Wu, P | Case report | China | 1 | 2.83 | 1 (100%) |
Conjunctivitis and eyelid dermatitis | Normal CBC, CRP, CK, liver measurements, ↑CK-MB, ↑LDH, ↓creatinine | Normal lung CT and x-ray | NR | NR |
| 112 | Wu, Q | Case series | China | 74 | 6.8 | 44 (60%) |
Asymptomatic (40.5%), cough (32.4%) and fever (27.0%) | Leukopenia (n=4), lymphopenia (n=4), ↑CRP (n=13), ↑PCT (n=2), ↑ESR (n=5); co-infection (n=26) | CT: ground glass opacities (n=9), atypical changes of bronchopneumonia and common viral pneumonia (n=28); normal (n=37) | All patients received interferon, Chinese medications, and antivirals; 27 patients received antibiotics | 1 |
| 113 | Wu, Z | Cross sectional | China | 965 | NR | NR | NR | NR | NR | NR | NR |
| 114 | Xia, W | Case series | China | 20 | NR | 13 (63%) |
Cough (n=13), fever (n=12), diarrhea (n=3), dyspnea (n=2), sore throat (n=1), fatigue (n=1) | 13 patients with elevated lymphocytes; 2 patients with elevated WBC | CT: consolidation (n=10), ground-glass opacities (n=12), shadow (n=4), nodules (n=3) | NR | NR |
| 115 | Xing, Y | Case series | China | 3 | NR | NR | Fever in all patients, gastrointestinal symptoms (n=1) | SARS-CoV-2 detectable in stool for 1-3 weeks after negative conversion in throat swabs | NR | NR | 0 |
| 116 | Xing, YH | Case series | China | 3 | 4.2 | 2 (67%) |
Fever (n=3), 1 patient had cough and diarrhea | Viral RNA remained detectable in stool for longer than 4 weeks, leukocytosis (n=3), ↑Plts (n=2), ↑PCT (n=1), ↑CRP (n=1), ↑LDH (n=1), ↑D-dimer (n=1) | CT: unilateral ground glass opacities (n=1), unilateral consolidation (n=1), normal (n=1); CXR: patchy shadows (n=1) | Interferon, ribavirin, and Chinese medications were given to all patients | 0 |
| 117 | Xu, Y | Case series | China | 10 | 7.54 | 6 (60%) |
Fever (n=7), cough (n=5), sore throat (n=4), diarrhea (n=3), rhinorrhea (n=2), asymptomatic (n=1) | WBC counts normal, neutropenia (n=4), lymphocytopenia (n=3), lymphocytosis (n=1), ↑PCT (n=5), ↑ESR (n=3),↑CRP (n=3), ↑LDH (n=2), ↑D-dimer (n=1), ↑ferritin (n=1), normal CK | NR | Interferon (n=10), antibiotics (n=1), IVIG (n=1) | NR |
| 118 | Yin, X | Case report | China | 1 | 9 | 1 (100%) |
Fever; no cough, sore throat, or nausea | Lymphopenia, ↑α hydroxybutyrate dehydrogenase, ↑CRP, ↑amyloid, normal PCT and CK | CXR: normal | Antipyretic | NR |
| 119 | Yu, N | Case report | China | 1 | 0.004 | NR | Dyspnea; no fever, cough, or diarrhea | NR | CXR: mild pneumonia | Observation | 1 |
| 120 | Zeng, L | Case report | China | 1 | 0.05 | 1 (100%) |
Sneezing, vomiting, lethargy, poor feeding | ↑lymphocytes, ↓neutrophils and procalcitonin | CT: bilateral enhanced texture and blurred shadows | NR | 1 |
| 121 | Zeng, Lingkong | Case series | China | 3 | 0.003 | 3 (100%) |
Fever (n=2), lethargy (n=2), shortness of breath and cyanosis (n=1), vomiting (n=1) | Leukocytosis (n=2), ↑PCT (n=1), ↑CK-MB (n=1), thrombocytopenia (n=1) | CT: pneumonia (n=3) | Mechanical ventilation (n=1), antibiotics (n=1) | 3 |
| 122 | Zhang, B | Case series | China | 46 | 8.75 | 29 (63%) |
Asymptomatic (n=22), cough (n=15), fever (n=10), rhinorrhea/nasal congestion (n=6), sore throat (n=4), myalgia/fatigue (n=3) | No leukopenia or lymphopenia | CXR: ground glass opacity (n=13), mixed ground glass opacity and consolidation (n=4), local patchy shadowing (n=1), consolidation (n=1) | Most treated with 1-3 antiviral drugs | 0 |
| 123 | Zhang, B2 | Case series | China | 3 | 9.3 | 2 (67%) |
Asymptomatic (n=2), crying (n=1), fever, cough, and malaise (n=1) | PCT normal (n=3), lymphocytosis (n=1), ↑CRP (n=2), ↑CK (n=1), ↑LDH (n=1) | CT: normal (n=2); CXR: bilateral pneumonia (n=1) | Two hospitalizations for all patients due to persistent SARS-CoV-2 positivity; oseltamivir (n=2), arbidol and lopinavir/ritonavir (n=2), oxygen (n=2), all received Chinese medication | 0 |
| 124 | Zhang, M | Case series* | China | 1 | 15 | 1 (100%) |
Low-grade fever and myalgia | NR | NR | NR | NR |
| 125 | Zhang, T | Case series | China | 3 | 7.7 | 3 (100%) |
Fever (n=2), rhinorrhea (n=2), cough (n=1) | Normal electrolytes, liver, and kidney function, normal PCT, LDH, and IL-6; 1 patient with elevated CRP (64.7 mg/L); immunologic profile normal, stool nucleic acid was still positive 10 days after clinical recovery | CT: ground glass opacities (n=2) | Interferon, Chinese medications, and vitamin C for all patients, 1 patient received antibiotics | 0 |
| 126 | Zhang, Y | Case report | China | 1 | 0.25 | 0 (0%) |
Fever and sputum production | Decreased neutrophil count; elevated CRP and platelet count, normal PCT | NR | Ambroxol and aerosolization | 0 |
| 127 | Zhang, Z | Case series | China | 4 | 0.02 | 3 (75%) |
Fever (n=2), shortness of breath (n=1), cough (n=1), vomiting (n=1), and 1 asymptomatic | NR | CT: increased lung markings (n=3) | Supportive | 0 |
| 128 | Zhao, W | Case series | China | 2 | 6.5 | NR | NR | NR | NR | NR | NR |
| 129 | Zheng, F | Case series | China | 25 | 5.1 | 14 (56%) |
Fever (n=13), cough (n=11), diarrhea (n=3), dyspnea (n=2), vomiting (n=2), abdominal pain (n=2), nasal congestion (n=2) | Median WBC, lymphocytes, CRP, CK within normal limits; lymphopenia (n=10), normal renal and coagulation profile (n=23) | CT: bilateral patchy shadows/consolidations (n=11), unilateral patchy shadows/consolidations (n=5), normal (n=8) | Antiviral therapy (n=12, included interferon, arbidol, oseltamivir, and/or lopinavir/ritonavir), 13 received antibiotics; 2 patients were intubated, and given corticosteroids and IVIG | 2 |
| 130 | Zhou, Y | Case series | China | 9 | 1.58 | 4 (44%) |
Asymptomatic (n=5), fever (n=4), cough (n=2), rhinorrhea (n=1) | normal WBC (n=7), lymphocytosis (n=6), ↑LDH (n=2 of 4 samples), ↑CRP (n=2 of 7 samples) | CT: ground-glass opacities (n=7), nodular morphology (n=6) | Interferon (n=9), lopinavir (n=6) | 0 |
| 131 | Zhu, L | Case series | China | 10 | 9 | 5 (50%) |
Fever (n=4), cough (n=3), headache (n=2), asymptomatic (n=4) | WBC, CRP and PCT normal in all children; ↑ALT (n=2) | CT: pneumonia (n=5) | Lopinavir/ritonavir (n=4), interferon (n=4), oseltamivir (n=1), antibiotics (n=1), oxygen (n=1), glucocorticoids and IVIG (n=0) | 0 |
Abbreviations: ABG-arterial blood gas; ASA-aminosalicylate; ALT- alanine aminotransferase; AST-aspartate aminotransferase; BUN-blood urea nitrogen; BNP-brain natriuretic peptide; CBC-complete blood count; CK-creatine kinase; CPAP-continuous positive airway pressure; Cr-creatinine; CRP-C-reactive protein; CT-computed tomography; CXR-chest radiograph; ESR-erythrocyte sedimentation rate; Hgb-hemoglobin; Ig-immunoglobulin; IL-interleukin; IVIG-intravenous immunoglobulin; LDH-lactate dehydrogenase; LFTs-liver function tests; LUS-lung ultrasound; NK-natural killer cell; NR-not reported; PCT-procalcitonin; Plts-platelets; PT-prothrombin time; PTT-partial thromboplastin time; RSV-respiratory syncytial virus; TNF-tumor necrosis factor. *One patient met our inclusion, but the publication was a case series.
Twenty of the publications pertained to the neonatal population and the ages extended from an extremely premature neonate at 26 weeks gestation to 20 years of age. The level of evidence for all of the studies was 5 (1 is highest, 5 is lowest) and the risk of bias scores were between 2 to 7 (1 is lowest, 9 is highest, refer to Appendix 2).
A total of 7780 COVID-19 positive children were included. Fifty six percent of the individuals were male (Table 2).
Table 2.
Patient characteristics, exposure status, and hospital stay.
| # Studies | # Patients | N (%) | |
|---|---|---|---|
| Male gender | 113 | 4640 | 2582 (55.6) |
| Mean age (years) | 116 | 4517 | 8.9 ± 0.5 |
| Exposure from family member | 94 | 1360 | 1028 (75.6) |
| Travel to/lived-in high-risk area | 84 | 962 | 689 (71.6) |
| NP/throat SARS-CoV-2 detection | 89 | 787 | 681 (86.5) |
| Positive fecal viral shedding | 31 | 321 | 67 (20.9) |
| Positive urine viral shedding | 22 | 54 | 2 (3.7) |
| Length of hospital stay (days) | 68 | 652 | 11.6 ± 0.3 |
| Intensive care unit admission | 88 | 3564 | 116 (3.3) |
Continuous data presented as Mean ± SD. NP-nasopharyngeal.
The mean age was 8·9 years (SD 0·5) and 75·6% of patients were exposed to a family member who was diagnosed with COVID-19. The most common method for detection of the virus was through nasopharyngeal or throat swab (86·5%). Need for intensive care unit observation or treatment was low (3·3%). Twenty studies (n=655 individuals) reported an underlying medical condition; COVID-19 positive children who were immunosuppressed or had a history of a respiratory or cardiac condition comprised the majority (65·%). Moreover, influenza and Mycoplasma were the most common co-infections (see Table 3).
Table 3.
Underlying medical conditions and co-infection.
| # Studies | # Patients | N (%) | |
|---|---|---|---|
| Underlying conditions | 20 | 655 | 233 (35.6) |
| Immunosuppression | 71 (30.5) | ||
| Respiratory | 49 (21.0) | ||
| Cardiovascular | 32 (13.7) | ||
| Medically complex/congenital malformations | 25 (10.7) | ||
| Not reported | 17 (7.3) | ||
| Hematologic | 8 (3.8) | ||
| Neurologic | 8 (3.4) | ||
| Obesity | 8 (3.4) | ||
| Prematurity | 5 (3.4) | ||
| Endocrine/metabolic | 5 (2.1) | ||
| Renal | 4 (1.7) | ||
| Gastrointestinal | 1 (0.5) | ||
| Co-infections | 35 | 1183 | 72 (5.6) |
| Bacterial | |||
| Mycoplasma pneumoniae | 42 (58.3) | ||
| Enterobacter sepsis | 2 (2.8) | ||
| Streptococcus pneumoniae | 1 (1.4) | ||
| Viral | |||
| Influenza virus A/B | 8 (11.1) | ||
| Respiratory syncytial virus | 7 (9.7) | ||
| Cytomegalovirus | 3 (4.2) | ||
| Epstein-Barr virus | 3 (4.2) | ||
| Adenovirus | 2 (2.8) | ||
| Human metapneumovirus | 2 (2.8) | ||
| Human parainfluenza virus | 2 (2.8) | ||
Table 4 summarizes clinical symptoms and imaging findings in COVID-19 confirmed pediatric patients. No symptoms were described in 456 of 2367 patients (19·3%), while the two most common symptoms were fever (59·1%), and cough (55·9%). While upper respiratory symptoms were characteristic of COVID-19, some patients presented with mild or often overlooked symptoms such as fatigue, abdominal pain, or decreased appetite [[14], [15]–16]. Table 4 also summates imaging findings. According to chest x-ray and computed tomography (CT), 23·6% and 18·9% had normal results, respectively. Patchy lesions were observed in 105 of 501 patients on chest radiography and bilateral ground glass opacities were the most frequent CT abnormality.
Table 4.
Clinical symptoms and imaging
| # Studies | # Patients | N (%) | |
|---|---|---|---|
| Clinical symptoms | |||
| Asymptomatic | 119 | 2367 | 456 (19.3) |
| Fever | 119 | 2445 | 1446 (59.1) |
| Cough | 119 | 2445 | 1367 (55.9) |
| Rhinorrhea, nasal congestion | 119 | 2445 | 488 (20.0) |
| Myalgia, fatigue | 119 | 2445 | 457 (18.7) |
| Sore throat | 119 | 2445 | 446 (18.2) |
| Shortness of breath, dyspnea | 119 | 2445 | 287 (11.7) |
| Abdominal pain, diarrhea | 119 | 2445 | 159 (6.5) |
| Vomiting, nausea | 119 | 2445 | 131 (5.4) |
| Headache, dizziness | 119 | 2445 | 104 (4.3) |
| Pharyngeal erythema | 119 | 2445 | 80 (3.3) |
| Decreased oral intake | 119 | 2445 | 42 (1.7) |
| Rash | 119 | 2445 | 6 (0.25) |
| Chest x-ray findings | |||
| Normal | 49 | 501 | 118 (23.6) |
| Patchy lesions | 49 | 501 | 105 (21.0) |
| Ground-glass opacity | 49 | 501 | 30 (6.0) |
| Consolidation | 49 | 501 | 12 (2.4) |
| Computed Tomography (CT) findings | |||
| Ground-glass opacity | 67 | 1115 | 367 (32.9) |
| Normal | 67 | 1115 | 211 (18.9) |
| Patchy lesions | 67 | 1115 | 117 (10.5) |
| Consolidation | 67 | 1115 | 72 (6.5) |
Complete blood counts were the most common laboratory results described (see Table 5). Overall, leukocytes were within normal values (7·1 × 103/μL), whereas neutrophils were mildly decreased (44·4%) while lymphocytes were marginally elevated (39·9%). Markers of liver and renal function were normal. Four serum inflammatory markers were above the mean: D-dimer, procalcitonin, creatine kinase, and interleukin-6.
Table 5.
Laboratory values.
| # Studies | # Patients | Mean (SD) | |
|---|---|---|---|
| Complete blood count | |||
| Leukocytes (103/µL) | 63 | 811 | 7.1 (0.3) |
| (normal range 4.0-12.0) | |||
| Neutrophils (%) | 43 | 512 | 44.4 (2.7) |
| (normal range 54-62) | |||
| Lymphocytes (%) | 52 | 672 | 39.9 (2.0) |
| (normal range 25-33) | |||
| Hemoglobin (g/dL) | 35 | 211 | 12.9 (0.9) |
| (normal range 11.5-14.5) | |||
| Platelets (103/µL) | 38 | 115 | 272.5 (8.5) |
| (normal range 150-450) | |||
| Liver and renal function | |||
| Creatinine (mg/dL) | 27 | 449 | 0.3 (0.0) |
| (normal range 0.22-0.59) | |||
| Aspartate aminotransferase (U/L) | 32 | 469 | 29.4 (2.2) |
| (normal range 15-50) | |||
| Alanine aminotransferase (U/L) | 35 | 656 | 19.5 (1.0) |
| (normal range 5-45) | |||
| Urea (mg/dL) | 12 | 227 | 4.6 (0.9) |
| (normal range 5-18) | |||
| Inflammatory markers | |||
| C-reactive protein (mg/L) | 45 | 643 | 9.4 (0.5) |
| (male normal range 0.6-7.9) | |||
| (female normal range 0.5-10.0) | |||
| D-dimer (mg/L)* | 16 | 285 | 0.7 (0.1) |
| (adult normal range <0.4) | |||
| Procalcitonin (ng/mL)† | 29 | 259 | 0.25 (0.0) |
| (normal range ≤0.15 ng/mL) | |||
| Lactate dehydrogenase (U/L) | 25 | 404 | 276.6 (25.9) |
| (normal range 150-500) | |||
| Creatine kinase (U/L) | 25 | 193 | 197.9 (23.1) |
| (adult normal range 5-130) | |||
| Fibrinogen (mg/dL)* | 7 | 179 | 224.2 (1.3) |
| (normal range 220–440) | |||
| ESR (mm/h)* | 7 | 134 | 14.1 (3.4) |
| (normal range 0-20) | |||
| Interleukin-6 (pg/mL) | 9 | 92 | 26.1 (3.7) |
| (normal range ≤1.8) | |||
| Ferritin (ng/mL) | 3 | 22 | 51.6 (13.2) |
| (normal range 10-60) |
Given that the mean (SD) in our pediatric population was 8.9 ± 0.5 years we provide the lowest to highest numbers presented in children with a similar age range when possible (data from Nelson Textbook of Pediatrics 2019). *Gregory's Pediatric Anesthesia 2012 5th edition.
Mayo clinic laboratories.
Sixty-six studies (n=614 individuals) provided information regarding treatments. Interferon was the most commonly administered drug (41·0%), followed by empiric antibiotics (20·2%). Of note, glucocorticoids, and intravenous immunoglobulin was used in 4·1% and 3·1% of patients, respectively. Complications we evaluated were rare and only described in 21 studies. There were 7 cases of kidney failure (0·09%), 19 cases of shock (0·24%), and 42 children were intubated (0·54%). More details on treatments provided and complications can be found in Table 6.
TABLE 6.
Treatments and complications
| # Studies | # Patients | N (%) | |
|---|---|---|---|
| Treatments | |||
| Interferon | 66 | 614 | 252 (41.0) |
| Antibiotics | 66 | 614 | 124 (20.2) |
| Remdesivir/unspecified antiviral | 66 | 614 | 134 (21.8) |
| Herbs/home remedies/other | 66 | 614 | 126 (20.5) |
| Lopinavir/ritonavir | 66 | 614 | 71 (11.6) |
| Oseltamivir | 66 | 614 | 53 (8.6) |
| Hydroxychloroquine | 66 | 614 | 48 (7.8) |
| Glucocorticoids | 66 | 614 | 25 (4.1) |
| Intravenous immunoglobulin | 66 | 614 | 19 (3.1) |
| Arbidol | 66 | 614 | 16 (2.6) |
| Ribavirin | 66 | 614 | 13 (2.1) |
| Tocilizumab | 66 | 614 | 9 (1.5) |
| Complications | |||
| Death | 131 | 7780 | 7 (0.09) |
| Mechanical ventilation | 131 | 7780 | 42 (0.54) |
| Shock | 131 | 7780 | 19 (0.24) |
| DIC | 131 | 7780 | 9 (0.12) |
| Kidney failure | 131 | 7780 | 9 (0.12) |
| Cardiac injury | 131 | 7780 | 8 (0.10) |
| MIS-C | 131 | 7780 | 11 (0.14) |
Eleven patients (0·14%) met the CDC's criteria for MIS-C [7]. Compared to control (n=14), children with severe inflammation were more likely to present with dyspnea (72·7% vs 28·6%), vomiting (45·5% vs. 7·1%), and diarrhea (45·5% vs. 21·4%). White blood cell counts were comparable between the groups; however, patients with MIS-C have significant lymphopenia (11·1% vs. 41·8%). No difference was noted in platelets or liver function markers. Serum lactate dehydrogenase and D-dimer were higher in children with MIS-C (p<0·05, details provided in Table 7). Also, patients with MIS-C had lower expression of circulating CD16+CD56+ natural killer cells. Imaging findings and treatments were comparable in MIS-C and non-MIS-C patients.
Table 7.
Comparison between covid-19 children with and without multisystem inflammatory syndrome in children (MIS-C).
| COVID-19 | MIS-C | ||
|---|---|---|---|
| Number of patients | 14 | 11 | NA |
| Age, years | 7.5 (1.8, 13.7) | 1.1 (0.7, 12.0) | 0.15 |
| Gender, male | 10 (71.4%) | 6 (54.5%) | 0.43 |
| Clinical characteristics | |||
| Fever | 10 (71.4%) | 10 (90.9%) | 0.34 |
| Cough | 8 (57.1%) | 6 (54.5%) | 1.00 |
| Dyspnea | 4 (28.6%) | 8 (72.7%) | 0.04 |
| Vomiting | 1 (7.1%) | 5 (45.5%) | 0.02 |
| Diarrhea | 3 (21.4%) | 5 (45.5%) | 0.02 |
| Underlying medical conditions | 1 (7.1%) | 3 (27.3%) | 0.14 |
| Laboratory parameters | |||
| White blood cell count (103/µL) | 7.8 (4.6, 8.3) | 9.0 (5.0, 11.3) | 0.23 |
| Neutrophils | 49.4% (31.4, 65.4) | 58.9% (55.3, 65) | 0.25 |
| Lymphocytes | 41.8% (22.4, 53.8) | 11.1% (5.9, 25.7) | <0.01 |
| Hemoglobin (g/dL) | 12.6 (2.3) | 12.1 (2.4) | 0.66 |
| Platelets (103/µL) | 250 (173, 301) | 193 (107, 251) | 0.22 |
| Aspartate aminotransferase (U/L) | 23.0 (17.0, 37.0) | 30.0 (18.8, 36.0) | 0.96 |
| Alanine aminotransferase (U/L) | 17.0 (11.0, 31.0) | 26.6 (12.0, 55.0) | 0.45 |
| Creatine kinase (U/L) | 77 (71, 113) | 106 (62, 380) | 0.45 |
| Lactate dehydrogenase (U/L) | 217 (203, 367) | 459 (380, 609) | <0.01 |
| C-reactive protein (mg/L) | 1.1 (0.5, 9.9) | 13.3 (1, 57.9) | 0.07 |
| Interleukin-2 (pg/mL)* | 2.6 (1.0) | 1.4 (0.3) | 0.06 |
| Interleukin-4 (pg/mL)* | 4.4 (1.5) | 2.8 (0.8) | 0.11 |
| Interleukin-6 (pg/mL)* | 14.3 (4.8, 9.0) | 118 (4.7, 25.4) | 0.81 |
| Interleukin-10 (pg/mL)* | 6.9 (4.8, 9.0) | 15.1 (4.7, 25) | 0.56 |
| Tumor necrosis alpha (pg/mL)* | 4.3 (3.2, 5.4) | 8.4 (1.4, 4.5) | 0.46 |
| Interferon gamma (pg/mL)* | 8.6 (5.9, 15) | 3.1 (1.5, 21) | 0.25 |
| CD16+CD56+* | 11.0% (5.1) | 4.2% (2.2) | 0.03 |
| CD3+* | 72.0% (14.4) | 60.0% (12.9) | 0.23 |
| CD4+* | 29.4% (3.8) | 34.7% (10.1) | 0.36 |
| D-dimer (mg/L)* | 0.3 (0.3, 0.5) | 40.3 (3.1, 11806) | <0.01 |
| Procalcitonin (ng/mL) | 0.09 (0.09, 0.13) | 0.11 (0.04, 0.83) | 0.72 |
| Imaging findings and treatment | |||
| Normal chest x-ray | 7 (50%) | 0 (0%) | 0.15 |
| Normal lung computed tomography | 5 (35.7%) | 0 (0%) | 0.47 |
| Interferon | 5 (35.7%) | 5 (45.5%) | 0.23 |
| Oseltamivir | 3 (21.4%) | 5 (45.5%) | 1.00 |
| Glucocorticoids | 2 (14.3%) | 6 (54.5%) | 0.13 |
| Intravenous immunoglobulin | 3 (21.4%) | 5 (45.5%) | 1.00 |
| Virazole | 3 (21.4%) | 4 (36.4%) | 1.00 |
| Tocilizumab | 0 (0%) | 2 (18.2%) | 0.49 |
Data are presented as mean (SD) or median (IQR). Student's t test, Wilcoxon rank sum, or Fisher's exact was conducted as appropriate. * denotes limited data was in at least one group (D-dimer in MIS-C=3; Interleukins and CDs had 4 in non-MIS-C group vs. 4-5 in MIS-C).
Discussion
Over the last 6 months, there have been over 6·4 million worldwide cases of SARS-CoV-2 infection and our knowledge of the disease and its epidemiologic and clinical characteristics continue to evolve [4]. However, since it was first reported in Wuhan city in December 2019, most studies have focused on symptomatic adults. In the presence of this rapidly emerging, novel infection, identification of clinical and laboratory characteristics in the pediatric population is essential to guide clinical care, predict disease severity, and determine prognosis. In this context, we performed the largest and most comprehensive systematic review of published studies involving pediatric patients with known COVID-19. Our systematic review summarized the clinical, laboratory and radiologic features of COVID-19 in neonates, children, and adolescents.
Our review also supports the findings by a recent systematic review by Castagnoli et al. [17] Their study included a total of 1,065 COVID-19 infected children and concluded that, by and large, the prognosis for children was excellent, demonstrated by only one death. Compared to that review and other COVID-19 pediatric systematic reviews, [[18], [19], [20], [21]–21] this manuscript has several key advantages: (1) we summarize 131 studies that includes 7780 children from 26 different countries, (2) this report synthesizes underlying pediatric medical conditions and delineates bacterial and viral coinfections, (3) we quantitatively describe clinical symptoms and imaging findings, (4) herein, we conglomerate the mean and standard deviation of frequently used laboratory analytes in COVID-19 positive children, (5) our report presents antiviral therapies by specific agents, and (6) our systematic review offers a preliminary comparison of patients with/without MIS-C.
Although SARS-CoV-2 infection was first identified in China, the United States has now amassed the highest number of confirmed cases [18]. Calculations made on June 4th, 2020 from the COVID-19 Dashboard by the Center for
Systems Science and Engineering at Johns Hopkins University indicate that China has 4·5% of total confirmed COVID-19 cases compared to the United States [4]. As expected, the most common vector for childhood infection is close contact to an affected family member or residing in an area with a high population of cases. Our findings align with the results of an April 2020 report by Dong et al, in which there was a clear trend that the disease spread rapidly from a Chinese province to surrounding provinces and cities in children from December to February [22]. Furthermore, Qiu and colleagues studied 36 pediatric COVID-19 positive patients in which ten patients (28%) were asymptomatic latent cases identified secondary to an adult family member who was infected, symptomatic, or traveled to an endemic area [23]. This lends concern that children, who may be asymptomatic, may play a role in community transmission of the virus.
Results from this systematic review echo findings describing milder symptoms in pediatric cases of SARS-CoV-2 infection [17,21]. For instance, the most common clinical manifestations we found were fever (59·1%), cough (55·9%), rhinorrhea (20·0%) and myalgia/fatigue (18·7%). Unlike adults, children rarely progressed to severe upper respiratory symptoms requiring intensive care unit admission [24,25]. Although transmission rates for SARS-CoV-2 are high, symptoms are less severe than SARS/Middle East Respiratory Syndrome (MERS) infection [26].
Serum inflammatory markers, specifically D-dimer, procalcitonin, creatine kinase, and interleukin-6, were consistently abnormal in the studies included in this review. Alterations to acute-phase infection-related biomarkers are corroborated in adult case series and meta-analyses [27,28]. However, we must take caution when interpreting these outcomes and await more robust, longitudinal laboratory analyses. Again, these blood analyses are non-specific and may merely represent a pro-inflammatory state induced by the virus [26].
In terms of imaging findings, we found that most patients had normal chest x-rays, a finding that is not surprising as most pediatric patients did not present with respiratory symptoms. Paralleling this review, a meta-analysis of CT features for COVID-19, showed that diffuse bilateral ground-glass opacities were the most common finding at all stages of disease [29,30]. Despite these promising associations, it is important to consider that radiologic manifestations from various pathogens may have a similar impression and should be ruled out. Co-infections with other respiratory illnesses including influenza and mycoplasma were described in 72 patients. As elegantly described by Cox and colleagues, most fatalities from the 1918 influenza outbreak were secondary to bacterial infection [31]. Thus, future reports should not only describe coinfections but also detail pertinent negatives. At present, our study had a low rate of reporting the infectious workup (26·7) of patients. Illustrating the importance, one of two patients that died in the study by Shekerdemian et al was due to gram negative sepsis in a child with comorbidities who developed end organ failure [32].
Although most children have an uneventful course, a present concern is an inflammatory cascade in pediatric patients with COVID-19 [8,9]. Clinical presentation includes an unremitting high fever, and includes systemic signs such as rash, conjunctivitis, and/or gastrointestinal symptoms. The case series of eight children from London required respiratory assistance, whether it was oxygen support (n=1), noninvasive ventilation (n=2) or intubation and mechanical ventilation (n=4) [8]. One patient was so ill that he required mechanical ventilation and extracorporeal membrane oxygenation. In addition, all required vasopressor support and demonstrated elevated levels of ferritin, D-dimers, troponin, procalcitonin, and C-reactive protein (CRP). Additionally, cardiac imaging showed ventricular dysfunction in five children. In another article, Italian investigators describe ten patients with MIS-C. Correspondingly, they describe patients manifesting with fever, diarrhea (n=6), and abnormal echocardiograms (n=6). Laboratory specifics showed elevated CRP, lymphopenia, thrombocytopenia, and elevated ferritin levels [9].
We found evidence of MIS-C features in 11 children who also presented with fever (n=11), dyspnea (n=8), and diarrhea (n=6). According to Riphagen and Verdoni, lymphopenia was marked in our cohort of patients, as well as increased levels of lactate dehydrogenase, CRP and D-dimer [8,9]. Despite low numbers we did observe an interesting lower level of CD16+CD56+ natural killer (NK) cells in patients with MIS-C. Both lymphopenia and a reduced number/activity of NK cells in adults has correlated with a more severe COVID-19 disease progression [[33], [34], [35]–36].
Little is known about the perinatal aspects of COVID-19, and there have been several reported cases of neonatal infection, suggesting a possible perinatal or vertical transmission during pregnancy [37]. However, in a report by Chen et al., all nine neonates born to COVID-19 positive mothers tested negative for the virus after cesarean delivery [38]. In another study by Zhang et al., 10 neonates from COVID-19 positive mother all tested negative for the infection [39]. Moreover, this is further supported by analysis of breast milk and placental pathologic specimens from COVID-19 positive mothers, which have returned negative for the virus [40,41]. Lastly, vertical transmission was not observed with either SARS-CoV-1 or in MERS-CoV;[41] therefore, it is unlikely that maternal vertical transmission during third trimester occurs, or is likely very rare. However, from the limited data published, we cannot determine the consequences of SARS-CoV-2 infection in early pregnancy and if it can be transmitted to the fetus and hinder organ development, malformations, growth abnormalities, or even lead to premature labor or spontaneous abortions [42,43]. Also, Dong et al communicated an alarming finding in which the proportion of severe and critical cases were higher in neonates when compared to the >16-year-old age group (10·6% vs. 3·0%) [44]. As a community, we must stay vigilant, practice social distancing, hand wash frequently, and be especially careful with our children who are at potentially higher risk for critical disease (e.g. multiple comorbidities, weakened immune systems, etc.).
There are several limitations to this review. First, many of the included studies were case reports or cases with low patient numbers. Second, the level of evidence for all the studies was low. Next, we unified the laboratory data to mean and standard deviation. There are inherent issues when using averages including the impact of outliers. We did not include suspected cases, which would allow for a direct comparison of symptoms, labs, imaging, and outcome data. Of concern, many of the studies were incomplete and did not include a comprehensive picture of the patients. Future studies should not generalize data (“CBC was normal”), or categorize laboratory values (i.e., number of patients with elevated CRP), or group therapies (i.e., patient received “antiviral therapy”), or display aggregate data between adults and children. If feasible, divide the symptoms, laboratory markers, and imaging characteristics by children vs. adults. A better understanding of COVID-19 requires access to data, even if it is provided in the appendix or supplementary section of the article. In this way, we will be able to identify the best biomarkers that can stratify disease severity and potential short- and long-term outcomes. Another limitation, is that we had a small number of patients that fit the criteria for MIS-C. Reasons for the small number of patients includes a lack of reporting all of the signs, symptoms, and laboratory markers necessary to make the diagnosis (especially duration of fever). Missing information for laboratory markers (D-dimer, interleukins, and CD%) hinders our preliminary findings. Lastly, the literature focusing on COVID-19 is very dynamic and growing rapidly and we expect the rates, especially for MIS-C, of our outcomes to change.
Contributors
Ansel Hoang-literature search, study design, data collection, data analysis, data interpretation, manuscript writing, risk of bias, tables. Kevin Chorath-literature search, study design, data collection, data interpretation, manuscript writing, risk of bias. Axel Moreira-literature search, study design, data collection, manuscript writing, data interpretation, risk of bias. Mary Evans-data collection, verifying data integrity, risk of bias. Finn Burmeister-Morton-data collection, verifying data integrity. Fiona Burmeister-data collection, verifying data integrity, risk of bias. Rija Naqvi-data collection, verifying data integrity, risk of bias. Matthew Petershack-data collection, risk of bias. Alvaro Moreira-literature search, study design, data collection, data analysis, data interpretation, manuscript writing, figure, tables, oversight.
Declaration of Competing Interest
None.
Acknowledgments
Funding sources: Parker B. Francis; Pilot grant 2R25-HL126140. Funding agencies had no role in the writing of the manuscript or the decision to submit.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100433.
Appendix. Supplementary materials
References
- 1.Du Z, Wang L, Cauchemez S. Risk for transportation of coronavirus disease from Wuhan to other cities in china. Emerg Infect Dis J. 2020;26:1049. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Q, Hu Y, dai Sr Z, Wu J, Xiao F, Wang J. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in Jingmen, Hubei. China. 2020;10 doi: 10.1101/2020.03.07.20031393. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucinotta D, Vanelli M. WHO declares covid-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns Hopkins University and Medicine. Coronavirus resource center. Coronavirus.jhu.edu/map.html (accessedJune3, 2020).
- 5.WHO. Coronavirus disease 2019 (COVID-19): situation report-28, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200217-sitrep-28-covid-19.pdf?sfvrsn=a19cf2ad_2(accessed May 13, 2020).
- 6.Wang E, Brar K. COVID-19 in children: an epidemiology study from China. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.04.024. April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Resources for Emergency Health Professionals. Health Alert Netw. 2020 https://emergency.cdc.gov/han/2020/han00432.asp (accessed June 3. [Google Scholar]
- 8.Riphagen S, Gomez X, Gonzales-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic Lancet2020; May 7. DOI:10.106/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed]
- 9.Verdoni L, Mazza A, Gerasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31103-X. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institutes of Health (2014) National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. Nhlbi.nih.gov/health-topics/study-quality-assessment-tools. June 3, 2020 (accessed. [Google Scholar]
- 11.Farrah K, Young K, Tunis M, Zhao L. Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev. 2019;1:280. doi: 10.1186/s13643-019-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95:2S–4S. [PubMed] [Google Scholar]
- 13.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus Disease 2019 in Children — United States, 2020. MMWR Morb Mortal Wkly Rep 2020February 12–April 2; 69:422-426. DOI:10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed]
- 15.Wang J, Wang D, Chen GC, Tao XW, Zeng LK. SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:211–214. doi: 10.7499/j.issn.1008-8830.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng L, Xia S, Yuan W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castagnoli R, Votto M, Licari A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1467. April 22. [DOI] [PubMed] [Google Scholar]
- 18.Souza T, Nadal J, Nogueira R, Pereira R, Brandao M. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol. 2020 doi: 10.1002/ppul.24885. June 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panahi L, Amiri M, Pouy S. Clinical characteristics of COVID-19 infection in newborns and pediatrics: a systematic review. Arch Acad Emerg Med. 2020;18:e50. [PMC free article] [PubMed] [Google Scholar]
- 20.Mustafa N, Selim L. Characterisation of COVID-19 pandemic in paediatric age group: a systematic review and meta-analysis. J Clin Virol. 2020 doi: 10.1016/j.jcv.2020.104395. May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludvigsson J. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y, Mo X, Hu Y. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in china. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. March 17. [DOI] [Google Scholar]
- 23.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang. China Observat Cohort Study Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30198-5. March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thabet F, Chehab M, Bafaqih H, Almohaimeed S. Middle east respiratory syndrome coronavirus in children. Saudi Med J. 2015;36:484–486. doi: 10.15537/smj.2015.4.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25948. April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Ji P, Pang J. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25884. April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar-Venugopal V, Mahajan V, Rajan S, et al. A systematic meta-analysis of CT features of covid-19: lessons from radiology, 2020; April 7. DOI:10.1101/2020.04.04.20052241.
- 31.Cox M, Loman N, Bogaert D, O'Grady J. Co-infections: potentially lethal and unexplored in COVID19. Lancet. 2020 doi: 10.1016/S2666-5247(20)30009-4. April 2410.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shekerdemian L, Mahmood N, Wolfe K, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr; May 11. DOI:10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed]
- 33.L Antonioli, Fornai M, Pellegrini C. Blandizzi C. NKG2A and COVID-19: another brick in the wall. Cell Mol Immunol. 2020;17:672–674. doi: 10.1038/s41423-020-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vabret N, Britton G, Gruber C. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan L. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020 doi: 10.1038/s41392-020-0159-1. April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang G, Kovalic AJ, Graber CJ. Prognostic value of leukocytosis and lymphopenia for coronavirus disease severity. Emerg Infect Dis. 2020 doi: 10.3201/edi2608.201160. May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong L, Tian J, He S. Possible vertical transmission of sars-cov-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Guo J, Wang C. Clinical characteristics, and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Jiang Y, Wei M. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020;55:E009. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 40.Zheng F, Liao C, Fan Q. Clinical characteristics of children with coronavirus disease 2019 in Hubei. China Curr Med Sci. 2020 doi: 10.1007/s11596-020-2172-6. March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S, Huang B, Luo DJ. [Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases] Zhonghua bing li xue za zhi. 2020;49:E005. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.02.017. February 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mimouni F, Lakshminrusimha S, Pearlman SA, Raju T, Gallagher PG, Mendlovic J. Perinatal aspects on the covid-19 pandemic: a practical resource for perinatal-neonatal specialists. J Perinatol. 2020;40:820–826. doi: 10.1038/s41372-020-0665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Y, X Mo, Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. March 13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.