Figure 4.
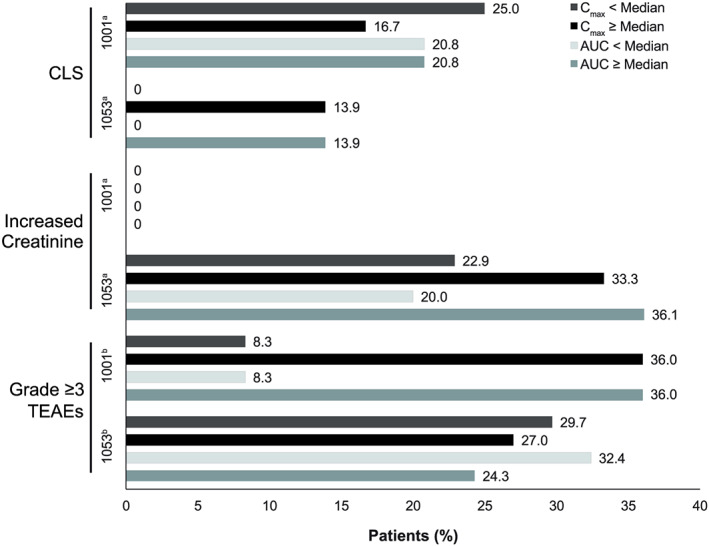
Incidence of selected adverse events, with events stratified by model‐predicted median Cmax and AUC in study 1001 and study 1053. aMedians for cycle 2 day 1: Study 1001, Cmax = 660.64 ng/mL; AUC = 2002 h ng/mL. For study 1053, Cmax = 423.1 ng/mL; AUC = 652.8 h ng/mL. bMedians for cycle 1 day 1: For Study 1001, Cmax = 286.74 ng/mL; AUC = 169.53 h ng/mL. For Study1053, Cmax = 188.89 ng/mL; AUC = 122.03 h ng/mL.
AUC, area under the moxetumomab pasudotox concentration–time curve; CLS, capillary leak syndrome; Cmax, maximum plasma drug concentration; Gr, grade; TEAE, treatment‐emergent adverse event
