Figure 1: Activation of platelets by HIVpp and influenza and proposed activation of platelet-Toll like receptor 7 (TLR7) by SARS-CoV-2.
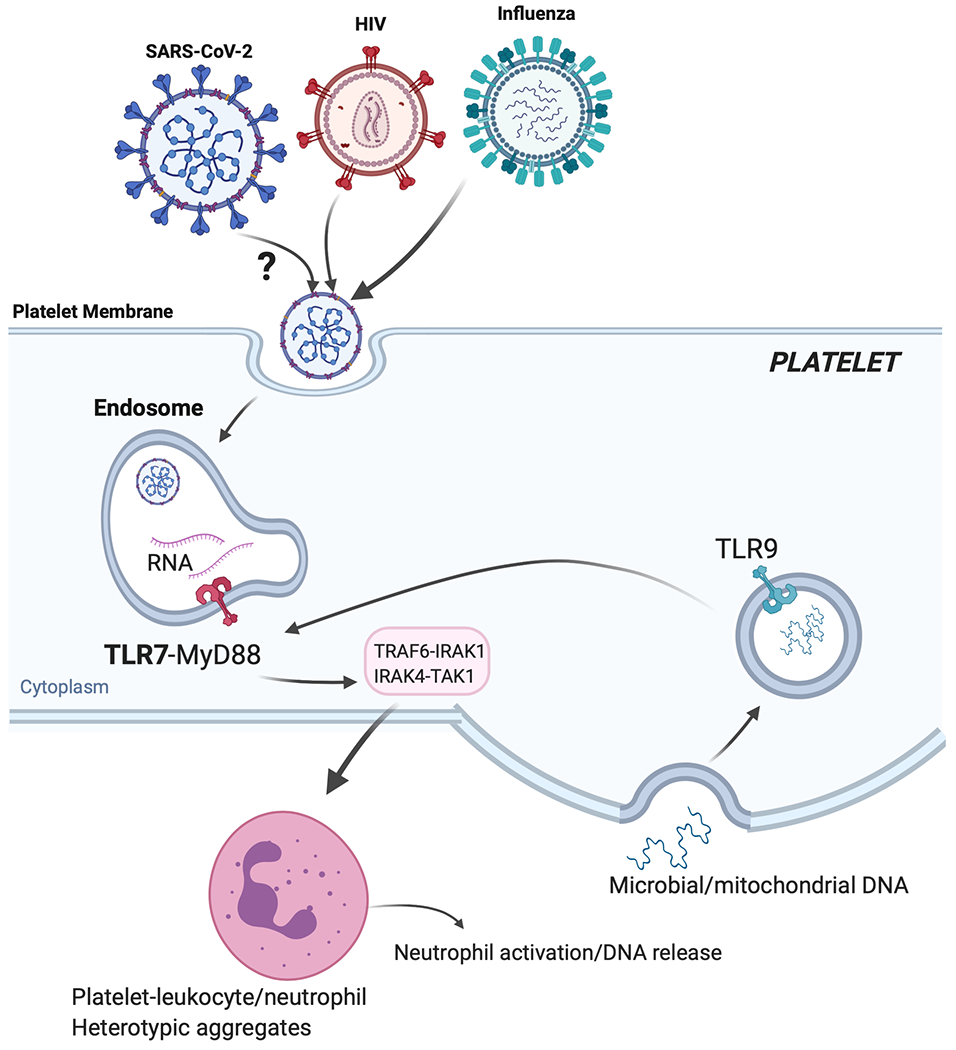
If SARS-CoV-2 (similarly to influenza or HIVpp) can be endocytosed by platelets, its degraded ssRNA would be first recognized by TLR7. Activation of TLR7 in a MyD88-IRAK-IKKb-SNAP23 dependent signaling would lead to P-selectin/CD40L surface expression and complement C3 release form α-granules. P-selectin and CD40L would mediate platelet-leukocyte interactions (predominantly neutrophils). C3 release would increase neutrophil-DNA release and ensures viral capture and removal. As viral infection progresses, C3-mediated netosis may be augmented by thrombin generated as a result of TF released from damaged tissue; thrombin will also directly increase thrombosis. During COVID19, TLR9, could also contribute to platelet activation and possible C3 release. TLR9 can be activated by mitochondrial-DNA and mitochondrial-DNA levels can be increased with tissue damage (or in the setting of obesity). Platelet-TLR9, similarly to TLR7, signals through the MyD88-IRAK-IKKb-SNAP23 pathway leading to granule release. It is not currently known if platelets can release C3 or netosis in a TLR9-dependent manner.
