Figure 4).
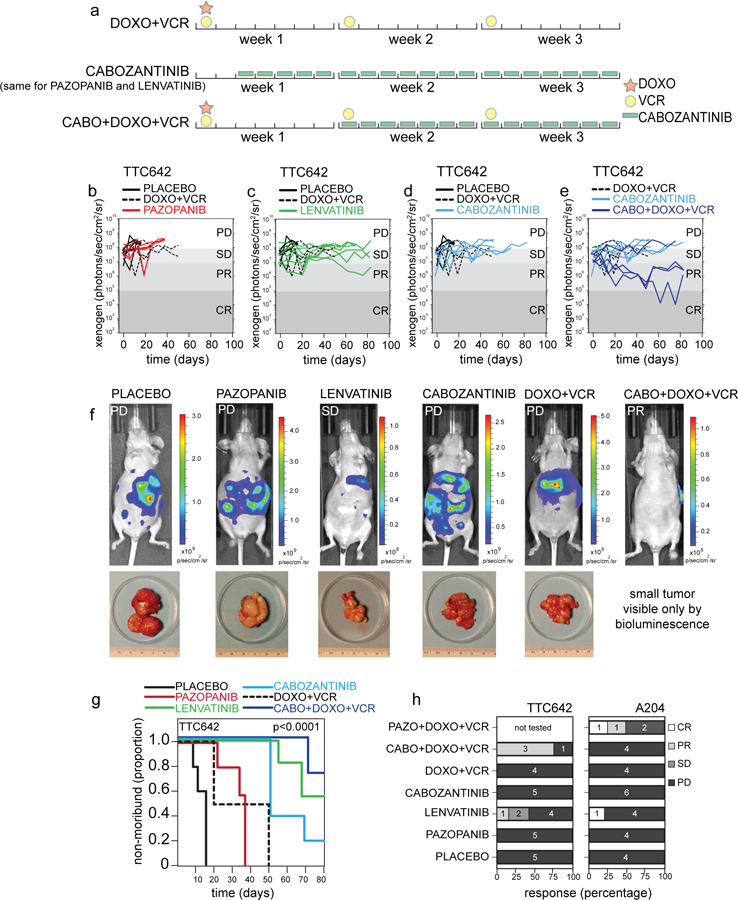
Preclinical testing of rhabdoid orthotopic xenografts.
a) Drug schedule selected for preclinical testing matched to relevant regimens used in patients. (b-e) Representative plot of tumor burden for TTC642 xenografts, as measured by bioluminescence over time, in a preclinical phase 2 study with the treatment groups listed compared to placebo control and DOXO+VCR (standard of care regimen used for rhabdoid tumor). Each line is a different mouse. f) Representative images and accompanying tumor micrographs from TTC642 xenograft mice with progressive disease in the placebo control, pazopanib, cabozantinib, and DOXO+VCR groups. Representative images of mice with stable disease in the lenvatinib group and a partial response shown for the CABO+DOXO+VCR treatment group. g) Survival curves for the TTC642 xenografts for the 6 indicated treatment groups, p-value determined by log-rank tests for each survival group. h) Histogram of the percentage of CR, PR, SD, and PD as determined by bioluminescence criteria for both TTC642 and A204 rhabdoid xenografts. The number of mice in each group is indicated. Abbreviations: CABO, cabozantinib; DOXO, doxorubicin; VCR, vincristine; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response; PAZO, pazopanib.
