Abstract
Exposure to wildland fire-related particulate matter (PM) causes adverse health outcomes. However, the direct links between cardiopulmonary health effects of wildland fire air pollution and specific biomass sources remain unclear. The purpose of this study was to investigate cardiopulmonary responses in rats following exposure to PM extracts collected from peat fire smoke. We hypothesized that peat smoke PM exposure would dose-dependently alter cardiopulmonary function. Male Sprague-Dawley rats (n = 8/group) were exposed to 35 μg (Lo PM) or 350 μg (Hi PM) of peat smoke PM extracts suspended in 200 μL of saline, or 200 μL of saline alone (Vehicle) via oropharyngeal aspiration (OA). We evaluated ventilatory effects of exposure immediately after OA in whole-body plethysmographs for 12 minutes. Ventilatory expiration times were the lowest in Hi PM exposed subjects at 6 minutes into recovery (p=0.01 vs. Lo PM, p=0.08 vs. Vehicle) and resolved shortly afterwards. The next day, we evaluated cardiovascular function in the same subjects via cardiac ultrasound under isoflurane anesthesia. Compared to Vehicle, Hi PM had 45% higher end systolic volume (p = 0.03) and 17% higher pulmonary artery blood flow acceleration/ejection time ratios, and both endpoints expressed significant increasing linear trends by dose (p = 0.01 and 0.02, respectively). In addition, linear trend analyses across doses (Vehicle, Lo PM, and Hi PM) detected an increase for end diastolic volume and decreases for ejection fraction and fractional shortening. These data suggest that exposure to peat smoke emissions modulates regulation of ventricular ejection and filling volumes, which could be related to altered blood flow in the pulmonary circulation. Moreover, early airway responses to peat smoke PM may point to irritant/autonomic mechanisms as potential drivers of later cardiovascular responses.
Keywords: wildfire, wildland fire, smoke, peat, biomass, particulate matter, heart function, ultrasound, echocardiography, airway irritation
INTRODUCTION
Wildland fire is a prominent source of air pollution (Landis et al. 2018) and has been associated with adverse cardiopulmonary health effects (Haikerwal et al. 2015; Reid et al. 2016). Due to expected warming and drying conditions, wildland fires are predicted to increase in both frequency and intensity, degrading air quality in more airsheds, and potentially affecting more people (Westerling et al. 2006; Abatzoglou and Williams 2016; Baker et al. 2018). Combustion emissions from wildland fires consist of complex mixtures of particulate matter (PM), gases, and volatile and semi-volatile organic compounds. Fine PM (<2.5 μm in diameter) represents one of the most extensively studied components of ambient air pollution, and is known to initiate autonomic and inflammatory responses in the lungs, heart, and blood vessels, and is strongly associated with the development and progression of cardiovascular disease (Brook et al. 2010; Cascio 2016; Chan et al. 2016; Argacha et al. 2017; McGuinn et al. 2017; Horne et al. 2018). However, little is known about the nature and composition of PM from wildland fire emissions and the role it plays in triggering negative health consequences.
Peat is a biomass comprised of decaying vegetation found in wetlands around the world. Though typically fire resistant when intact and undisturbed, carbon-rich peatlands can become a unique fuel source prone to slow, smoldering burns deep in the soil that can last from months to years and release unusually high amounts of fine PM, driving serious health concerns (Page and Hooijer 2016). Peat fires are expected to rise in the coming years (Laurance and Laurance 2015) and represent a global concern given the massive acreage of peatlands burned annually for agricultural purposes in Southeast Asia, which can impact air quality over long distances across neighboring countries (Gaveau et al. 2014; Hayasaka et al. 2014; Urbancok et al. 2017). In eastern North Carolina (USA), the peat fires of Pocosin Lake in 2008 and Pains Bay in 2011 were both associated with increased hospitalizations in affected counties, all attributable to cardiovascular and pulmonary complications (Rappold et al. 2011; Tinling et al. 2016). Despite the epidemiological evidence associating peat smoke inhalation with adverse health outcomes, the specific biological responses driving these effects are unclear.
The purpose of this study was to investigate the cardiopulmonary responses after intrapulmonary exposure to peat biomass smoke condensates in rats. This study used a glass tube furnace system to generate peat smoke that allowed for automated combustion under flaming or smoldering burn conditions and has been previously used to compare health effects of different biomass fuel sources, including peat (Kim et al. 2018). The condensates, which consisted of particulate matter and semivolatile components of peat smoke, were collected using a multistage cryotrap system. We hypothesized that exposure would dose-dependently alter cardiopulmonary function. To test this, we exposed rats to either a low or high concentration of peat biomass smoke PM extracts in saline, or to saline alone, by oropharyngeal aspiration (OA). Breathing parameters were assessed immediately following exposure, while pulmonary and systemic markers of inflammation/injury and cardiovascular function measured using high frequency ultrasound were assessed the following day.
MATERIALS AND METHODS
Ethical Statement:
Use of animals in this study complied completely with laboratory animal use protocols approved by the Institutional Animal Care and Use Committee at the U.S. Environmental Protection Agency Research Facility in Research Triangle Park, NC.
Experimental Design:
The study was designed to test potential sensory/airway irritation responses immediately following oropharyngeal aspiration of peat biomass smoke extract by measuring ventilatory function and heart responses 24 hours following exposure in rats. Airway irritation responses were tested via whole-body plethysmography in rats. Heart function by cardiac ultrasound was assessed in the same rats at 24 hours after exposure while under isoflurane anesthesia. Given that isoflurane depresses heart rate and that many heart function parameters are influenced by heart rate, we excluded any ultrasound data when average heart rate was <300 beats/minute or >400 beats/minute. We used a high dose of 350 μg peat biomass smoke PM extract (Hi PM), a low dose of 35 μg peat PM smoke extract (Lo PM), and a saline vehicle control (Vehicle).
Sample Size Analysis:
We used R Studio (version 3.1.2) to conduct sample size analysis with the ‘pwr’ package (https://cran.r-project.org/web/packages/pwr/pwr.pdf) and ‘pwr.anova.test’ command. We used data from a previous study (Thompson et al. 2017) showing slight changes in myocardial performance index (i.e. Tei index; see Tei et al. 1995 for description) as a basis for biological relevance in effect size estimations. Thus, we powered the study to be sensitive enough to capture a 0.10 increase in Tei index, which was the approximate increase observed in the previous study. The largest standard deviation (SD) reported in that study was 0.07. Cohen’s equation (Cohen 1988) was used to calculate effect size (d = effect range/control SD = 0.10/0.07 =1.43) and effect size index (f = 0.5 * d = 0.5*1.43 = 0.72). For group size (n) determinations, we set k (number of groups) = 3 since we were planning for 2 doses of peat smoke extract and 1 saline vehicle group, significance level = 0.05, and power = 0.8. The sample size analysis yielded n = 8.
Animals:
Male Sprague-Dawley rats were used for this study at 15-17 weeks of age and 469 ± 56 g of body mass (Charles River, Wilmington, MA, USA). Rats were housed at 23 ± 1°C on 12-hour light/dark cycles, 2/cage, with free access to food and water ad libitum in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility of the National Health and Environmental Effects Research Laboratory of the U.S. Environmental Protection Agency. Subjects were acclimated for at least 10 days prior to the study and were treated humanely throughout the entire procedure.
Peat Biomass Combustion and Sample Collection:
Peat biomass (15 g) collected from coastal plain of NC was combusted for 1 hour at approximately 640°C inside a quartz tube equipped with a ring furnace (Kim et al. 2018). Smoke flow from the tube furnace was collected in a multistage cryotrap system consisting of sequential impingers held at −10°C, −50°C, and −70°C.
Peat Smoke Particle Extraction and Suspension:
Peat smoke PM and semi-volatile compounds from the 3 impingers were combined and extracted into acetone. 3.5 mg of PM per mL of acetone was slowly mixed with 1 mL of saline and then the acetone was evaporated under nitrogen gas, leaving behind a residual mass suspended in saline at 3.5 mg/mL. The PM-saline suspensions were diluted further with saline to the final stock concentrations of 1.75 mg/mL saline (Hi PM) and 175 μg/mL saline (Lo PM). Stocks were stored at −80°C until use and vortexed thoroughly after thawing at the time of aspiration procedures.
Oropharyngeal Aspiration:
Rats were anesthetized under 3% isoflurane in 0.8-1.0 L/min medical grade oxygen until respiration became notably slower. Rats were briefly secured and suspended by their incisors and the tongue was extended with padded forceps to impede ingestion of aspirate suspensions. A micropipette was used to deliver a 200 μL bolus of saline, Lo PM suspension (35 μg total mass), or Hi PM suspension (350 μg total mass) into the opening of the oropharynx. The tongue was held in the extended position until the entire bolus was aspirated. Rats were moved immediately to whole-body plethysmographs for ventilatory assessment.
Whole-body Plethysmography:
Following PM exposure, ventilatory data were recorded continuously in whole-body plethysmography chambers (model PLY3213, Buxco Electronics, Sharon, CT) for 12 minutes. Each chamber contains a built-in reference chamber for measuring respiration-induced pressure fluctuations. Subjects were acclimated to plethysmographs for one hour on two separate days prior to exposure and measurement. A bias flow regulator delivered fresh air (1.8 L/min) to each cylindrical chamber, preventing CO2 buildup within the chamber. Data were channeled to computer with Emka iox2 software (Emka Technologies, Montreal, Canada) that calculated and recorded respiratory parameters by one-minute averages. After plethysmography, subjects were returned to their home cages for 24 hours.
Cardiac Ultrasound:
Rats were anesthetized with isoflurane at 24 hours after exposure. Fur was removed from the anterior exterior thorax using clippers and a depilatory agent. Subjects were positioned in dorsal recumbency for ultrasound. Electrocardiogram and respiratory signals were recorded via paw electrodes in Lead II configuration. To minimize the effect of heart rate on heart function, heart rates were targeted to 350 beats/minute by titrating anesthesia. A Vevo2100 and MS-201 transducer (FUJIFILM VisualSonics Inc., Toronto, Canada) were used to obtain parasternal long axis views of the left ventricle in M-mode (15 MHz) for functional measurements and pulsed wave Doppler (12.5 MHz) of pulmonary artery and transmitral blood flow. A total of 6 cardiac cycles were analyzed for every measurement using Vevo® LAB software (version 1.7.0; FUJIFILM VisualSonics Inc.). Complete blinding was used for the sonographer and during the subsequent analyses.
Necropsy:
Immediately following ultrasound, subjects were given an intraperitoneal injection of sodium pentobarbital/phenytoin (200/25 mg/kg) and allowed to enter surgical anesthesia. When unresponsive to toe pinch, a laparotomy was performed and blood was collected in serum separator tubes from the inferior vena cava and placed on ice. Next thoracotomy and tracheotomy was performed for right lung bronchoalveolar lavage (BAL). To do so the left main bronchus was tied off and 5 mL/kg of Hanks Balanced Salt Solution (HBSS, Gibco, #14175, Gaithersburg, MA, USA) was infused into the right lung, aspirated, collected, and placed on ice. The lavage was repeated a second time with fresh HBSS.
Serum and Lavage Sample Analyses:
Blood and BAL samples were centrifuged at 1500 x g at 4°C for 10 minutes. Serum and BAL supernatant was collected and snap frozen in liquid nitrogen. Cell pellets from BAL were resuspended and used to determine total cell counts using a Z1 Beckman-Coulter Counter (Beckman-Coulter Inc., Miami, FL). A second aliquot of the resuspended BAL pellet was centrifuged (Shandon 3 Cytospin, Pittsburgh, PA) to prepare cell differential slides. BAL slides were dried at room temperature and stained with Leukostat (Fisher Scientific, Pittsburgh, PA, USA). Macrophages, neutrophils, lymphocytes, and eosinophils were counted using light microscopy with at least 200 cells counted on each slide. Serum and BAL fluid samples were analyzed for biomarkers of inflammation, oxidative stress, and metabolic dysfunction using commercially available kits, modified for use on a Konelab Arena 30 Clinical Chemical Analyzer (Thermo Chemical Lab Systems, Espoo, Finland). For information on specific assays, please see the supplemental materials.
Statistics:
Data were analyzed and graphed with Graphpad Prism 6 software (version 6.07, La Jolla, CA, USA). Plethysmography data xy plots in Figure 1A and 1C are one-minute averages presented as mean ± standard error of the mean (SEM) for clarity and tested by Repeated Measures ANOVA with Bonferroni’s post-test. All other data are mean ± standard deviation (SD) or displayed as box and whisker plots with all data points shown, box edges identifying the interquartile range, middle lines identifying the median, “+” symbols identifying the mean, and whiskers identifying the minimum and maximum data values. These data are compared by one-way ANOVA with Tukey’s multiplicity adjusted post-test and a linear trend analysis (ANOVA). Statistical significance was taken as p < 0.05 but p-values less than 0.1 are provided for clarity.
Figure 1. Ventilatory changes minutes after exposure to peat smoke PM.
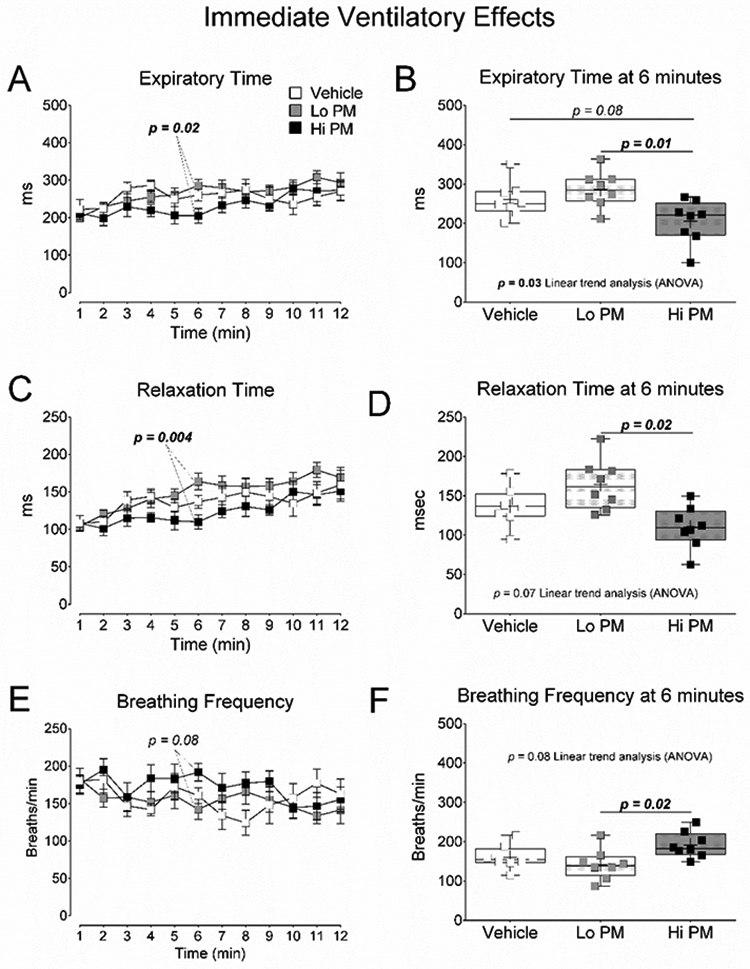
(A) One-minute averages of expiratory time during the 12 minutes of ventilatory assessment. (B) Average expiratory time at 6 minutes. (C) One-minute averages of relaxation time during the 12 minutes of ventilatory assessment. (D) Average relaxation time at 6 minutes. (E) One-minute averages of breathing frequency during the 12 minutes of ventilatory assessment. (F) Average breathing frequency at 6 minutes. A, C, and E are mean ± SEM of one-minute averages tested by Repeated Measures ANOVA with Bonferroni’s post-test. B, D, and F were tested by One Way ANOVA with Tukey’s and linear trend analysis post-tests. p-values less than 0.1 are provided for clarity.
RESULTS
Peat Smoke Extracts:
Condensates collected from peat smoke emissions have previously been characterized (Kim et al. 2018). Details about the combustion atmosphere characteristics and the composition of the condensates collected for this study are provided in Table 1. In short, during peat combustion the modified combustion efficiency (100 × ΔCO2/(ΔCO2+ΔCO)) was ~97%. Particle concentrations were ~15 mg/m3 with particle diameters ~0.89 μm. About 43% of the peat smoke condensate was organic carbon, 34% were ionic species, 13% were inorganic elements, and 10% was unidentified. Of the organic carbon fraction, about 2% was levoglucosan, <1% were N-alkanes, <1% were polyaromatic hydrocarbons, and about 97% was unidentified.
Table 1.
Peat Smoke Characterization1)
| Smoke Emission Characteristics | ||
|---|---|---|
| MCE | % | 97 |
| PM diameter | μm | 0.89 |
| CO | ppm | 159 |
| CO2 | ppm | 5042 |
| [PM] | mg/m3 | 15 |
| Emission Factor (g/kg fuel) | ||
| CO | g/kg fuel | 33 |
| CO2 | g/kg fuel | 1777 |
| PM | g/kg fuel | 1 |
| Condensate Characteristics | ||
| General Chemistry | ||
| Organic Carbon | % | 43 |
| Elemental Carbon | % | <1 |
| Inorganic Elements | % | 13 |
| Ionic Species | % | 34 |
| Unidentified | % | 10 |
| Organic Fraction | ||
| Levoglucosan | % | 2 |
| Methoxyphenols | % | <1 |
| N-Alkanes | % | <1 |
| Polyaromatic Hydrocarbons | % | <1 |
| Unidentified | % | 97 |
Abbreviations: MCE – modified combustion efficiency (100 × ΔCO2/(ΔCO2+ΔCO)), PM – particulate matter, ppm – parts per million
Data are obtained from Kim et al. (2018)
Overt Toxicity:
Exposure to peat smoke PM produced no signs of overt toxicity and no changes in subject weights (g ± SD) one day after exposure: 481 ± 39, 480 ± 40, and 476 ± 42 in the Vehicle, Lo PM, and Hi PM, respectively (n = 8).
Ventilatory Responses:
Ventilatory responses immediately after PM exposure are reported in Figure 1 and Table 2. Subjects from the Hi PM group displayed the lowest single-minute mean expiratory (Figure 1A and 1B) and relaxation times (Figure 1C and 1D), and highest single-minute mean breathing frequency (Figure 1E and 1F), 6 minutes into the ventilatory assessment. The Hi PM group also had the lowest 12-minute average for relaxation time (TR) reported in Table 2. In all three cases, data from the Hi PM group were significantly lower (p < 0.05) than data from the Lo PM group but not when compared to data from the Vehicle group.
Table 2.
Ventilatory Plethysmography Data
| Means for 12-minute Ventilatory Assessment | ||||
|---|---|---|---|---|
| Vehicle | Lo PM | Hi PM | ||
| BF | Breaths/minute | 157 ± 21 | 154 ± 23 | 172 ± 20 |
| TV | mL | 1.7 ± 0.3 | 1.8 ± 0.1 | 1.7 ± 0.1 |
| MV | mL/minute | 275 ± 46 | 279 ± 37 | 304 ± 44 |
| TI | ms | 157 ± 22 | 170 ± 24 | 159 ± 30 |
| TE | ms | 256 ± 25 | 265 ± 26 | 234 ± 36 |
| TR | ms | 137 ± 23 | 149 ± 15 | 124 ± 17 † |
| PIF | mL/second | 17.9 ± 3.3 | 17.9 ± 2.3 | 18.9 ± 2.6 |
| PEF | mL/second | 16.5 ± 2.0 | 16.0 ± 2.0 | 18.5 ± 2.6 |
| Pause | (TE-TR)/TR | 0.90 ± 0.23 | 0.80 ± 0.11 | 0.89 ± 0.14 |
| PENH | (PEF/PIF x Pause) | 0.92 ± 0.28 | 0.79 ± 0.21 | 0.96 ± 0.28 |
| Mean ± SD | ||||
p<0.05 vs. Lo PM by ANOVA with Tukey’s post-test
Abbreviations: BF – breathing frequency, TV – tidal volume, MV – minute ventilation, TI – inspiratory time, TE – expiratory time, TR – relaxation time, PIF – peak inspiratory flow, PEF – peak expiratory flow, PENH – enhanced pause
Heart Function:
Cardiac M-mode ultrasound was conducted to evaluate heart function one day after exposure to peat smoke PM. Heart function data are reported in Figure 2 and Table 3. End systolic volume (Figure 2A) was significantly (p < 0.05) elevated in the Hi PM group compared to end systolic volume in the Vehicle group. The data for end systolic volume also produced a significant increasing linear trend (ANOVA) with PM concentration. End diastolic volume (Figure 2B) showed a similar significant increasing linear trend (ANOVA) with PM concentration but was not statistically different between groups. The same was true for ejection fraction (Figure 2C) and fractional shortening (Figure 2D).
Figure 2. Changes in left ventricular volume regulation one day after exposure to peat smoke PM.
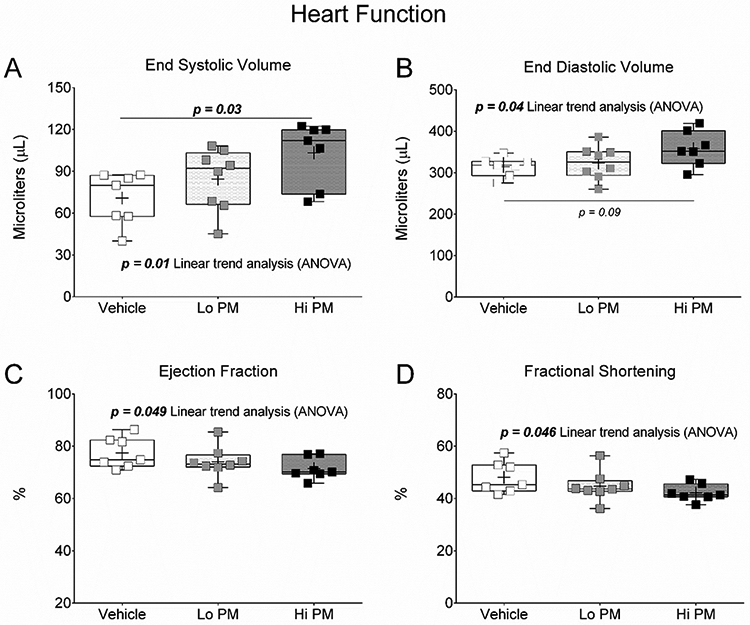
(A) End systolic volume. (B) End diastolic volume. (C) Ejection fraction. (D) Fractional shortening. Data were tested by One Way ANOVA with Tukey’s and linear trend analysis post-tests. p-values less than 0.1 are provided for clarity.
Table 3.
Heart Ultrasound Data
| General Cardiac Function | |||||
|---|---|---|---|---|---|
| Vehicle | Lo PM | Hi PM | Linear (p < 0.05) | ||
| HR | Beats/minute | 364 ± 16 | 338 ± 18 * | 368 ± 22 † | No |
| ESV | Microliters | 71 ± 19 | 84 ± 22 | 103 ± 23 * | ↑ |
| EDV | Microliters | 315 ± 24 | 324 ± 40 | 358 ± 43 | ↑ |
| SV | Microliters | 244 ± 25 | 239 ± 33 | 254 ± 26 | No |
| CO | mL/minute | 89 ± 10 | 81 ± 13 | 94 ± 7 | No |
| EF | % | 78 ± 6 | 74 ± 6 | 71 ± 4 | ↓ |
| FS | % | 48 ± 6 | 45 ± 6 | 42 ± 3 | ↓ |
| Mean ± SD | |||||
| Pulmonary Artery Blood Flow | |||||
| PAT/PET | : | 0.42 ± 0.07 | 0.45 ± 0.03 | 0.49 ± 0.06 * | ↑ |
| Mean ± SD | |||||
| Transmitral Blood Flow | |||||
| Performance | Tei index | 0.45 ± 0.1 | 0.47 ± 0.1 | 0.47 ± 0.1 | No |
| IVCT | %CC | 7.1 ± 2.3 | 7.1 ± 3.1 | 7.8 ± 2.2 | No |
| AET | %CC | 38.4 ± 2.5 | 37.2 ± 4.4 | 39.8 ± 3.8 | No |
| IVRT | %CC | 10.3 ± 1.1 | 10.0 ± 1.0 | 10.1 ± 0.6 | No |
| Mean ± SD | |||||
p<0.05 vs. Vehicle
p<0.05 vs. Lo PM by ANOVA with Tukey’s post-test, ↑increasing or ↓decreasing linear trend analysis (p<0.05)
Abbreviations: HR – heart rate, ESV – end systolic volume, EDV – end diastolic volume, SV – stroke volume, CO – cardiac output, EF – ejection fraction, FS – fractional shortening, PAT – pulmonary artery blood flow acceleration time, PET – pulmonary artery blood flow ejection time, IVCT – isovolumic contraction time, AET – aortic ejection time, IVRT – isovolumic relaxation time, %CC – percent of cardiac cycle
Cardiac Hemodynamics:
Pulsed-wave Doppler of pulmonary artery and transmitral blood flow was conducted one day after exposure to peat smoke PM to evaluate hemodynamics of the right and left ventricle, respectively. Data from these endpoints are presented in Figure 3 and Table 3. As was seen with end systolic volume, pulmonary artery blood flow acceleration/ejection time ratio was significantly (p < 0.05) elevated in the Hi PM group compared to acceleration/ejection time ratio in the Vehicle group (Figure 3). The data for pulmonary artery blood flow acceleration/ejection time ratio also produced a significant linear trend (ANOVA), increasing with PM concentration. Results from transmitral blood flow analysis were not statistically different between groups and did not produce any significant linear trends (Table 3).
Figure 3. Changes in pulmonary artery hemodynamics one day after exposure to peat smoke PM.
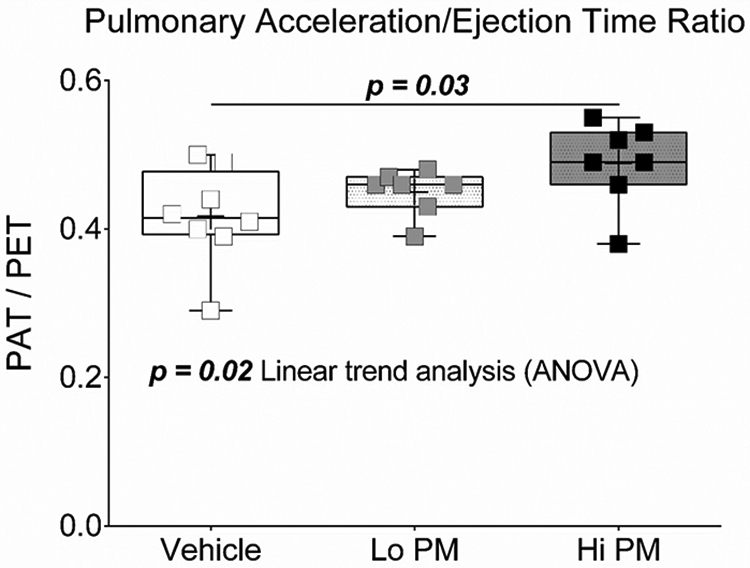
Data were tested by One Way ANOVA with Tukey’s post-test.
Biomarker Analyses:
Serum was analyzed for various biomarkers of metabolic dysfunction and tissue injury. Selected results are presented in Supplemental Figure S1. We also analyzed BAL fluid for various biomarkers of lung inflammation and oxidative stress. Differential cell counts are provided in Table 4 and quantification of super oxide dismutase is reported in Supplemental Figure S2. None of the factors from the serum and BAL analyses were statistically different but we have displayed some near significant p-values for clarity.
Table 4.
Bronchoalveolar Lavage Analyses
| Differential Cell Counts | ||||
|---|---|---|---|---|
| Vehicle | Lo PM | Hi PM | ||
| Total Cells | x 104 | 16.4 ± 6.7 | 12.2 ± 4.8 | 10.5 ± 3.7 |
| Macrophages | x 104 | 12.8 ± 6.3 | 10.5 ± 4.3 | 9.0 ± 2.8 |
| Neutrophils | x 104 | 2.8 ± 3.5 | 1.3 ± 1.7 | 1.1 ± 1.8 |
| Lymphocytes | x 104 | 0.7 ± 0.5 | 0.4 ± 0.3 | 0.4 ± 0.2 |
| Eosinophils | x 104 | 0.1 ± 0.2 | 0.0 ± 0.1 | 0.0 ± 0.0 |
| Mean ± SD | ||||
DISCUSSION
This study shows that airway exposure to extracts from peat smoke can induce transient tachypnea within minutes of exposure and can alter the regulation of left ventricular volume in the heart and pulmonary artery hemodynamics one day later. Such responses may render subjects vulnerable to subsequent stressors in the short term and/or, with repeated exposure over the long term, set the stage for development or progression of cardiovascular disease. However, our primary intent was to determine if exposure to peat smoke constituents would produce changes in cardiovascular physiology after first producing airway irritation. By utilizing the extract aspiration method of exposure followed immediately by plethysmography, we could instantaneously deliver the peat smoke constituents into the airways and begin recording ventilatory patterns to capture evidence of rapid-onset irritant responses. Providing support showing that exposure to air pollutants results in early, or even short-lived and transient, airway irritation in conjunction with alterations in heart function strengthens the plausibility that the latter biological response may indeed be precipitated by the first.
Constituents found in peat fire smoke appear to trigger irritant responses. Approximately six minutes after peat smoke extract exposure, rats had reductions in pulmonary expiratory time and relaxation time, resulting in an increase in breathing frequency between subjects exposed to high and low concentrations of the peat smoke extract. These changes are consistent with the sudden tachypnea reported in Sprague Dawley rats exposed to a single breath of Lauan wood smoke (Kou and Lai 1994). We suspect that these brief changes in ventilatory pattern shortly after exposure reflect the presence of pulmonary (i.e. lower airway) irritation, as has been described in BALB/c mice during ozone exposure (Nielsen et al. 1999). Airway irritant responses are thought to trigger changes in autonomic nervous system tone, which is one of the postulated mechanisms for subsequent cardiovascular events associated with air pollution (Perez et al. 2015; Cascio 2016; Kelly and Fussell 2017). In fact, cardiovascular responses to diesel exhaust have been previously linked to sensory nerve activation via transient receptor cation channel A1 (Hazari et al. 2011). Polyaromatic hydrocarbons implicated in diesel exhaust PM-dependent activation of transient receptor potential A1 on sensory C-fibers (Robinson et al. 2018) are also known constituents of peat smoke PM (Kim et al. 2018).
Secondly, exposure to constituents of peat fire smoke can alter the regulation of left ventricular volume. Particularly, we found increased left ventricular end systolic volume one day after exposure to peat smoke extracts. This may reflect diminished myocardial contractility and/or mechanosensation within the heart. According to the Frank-Starling mechanism, myocardial contractility increases proportionally to the amount of heart muscle displacement (i.e. stretch) caused by the volume of blood entering the ventricles from the atria, effectively ejecting any excess filling volume (Sequeira and van der Velden 2017). PM exposure has been linked to reductions in cardiac contractility (Simkhovich et al. 2008) and/or heart failure (Chan et al. 2016), in in vivo (Carll et al. 2015), ex vivo (Kurhanewicz et al. 2017), and in vitro models (Gorr et al. 2015). Moreover, peat smoke exposure was associated with an increase in emergency department visits due to cardiopulmonary symptoms and heart failure after a peat bog fire North Carolina in 2008 (Rappold et al. 2011). Ultrafine PM from peat fire smoke was also linked to impaired recovery of post-ischemic contractility in isolated hearts collected from exposed mice (Kim et al. 2014). If repeated peat smoke exposure results in sustained increases in end systolic volume, left ventricular dilation, cardiomyopathy, and heart failure could result, or lead to the worsening of similar pre-existing conditions.
Lastly, peat smoke extract exposure can alter pulmonary artery hemodynamics. We found that pulmonary artery acceleration/ejection time ratio increased after exposure, suggesting reduced pulmonary vascular resistance and/or pressure. Under ideal conditions the pulmonary arterial bed diverts blood away from poorly ventilated alveoli toward capillaries with higher O2 tensions, a physiological response known as hypoxic pulmonary vasoconstriction (Sommer et al. 2016). Pulmonary artery endothelial cells have been shown to be susceptible to oxidative stress induced by fine PM exposure in vitro (Deweirdt et al. 2017), possibly blunting hypoxic pulmonary vasoconstriction. In fact, vascular endothelial dysfunction is broadly reported following exposure to varying types of air pollution (Argacha et al. 2017; Kelly and Fussell 2017). Such pulmonary vascular responses to air pollution exposure may cause a loss of ventilation-perfusion matching, resulting in a secondary reduction in pulmonary arterial resistance. Alternatively, exposure to the peat smoke PM may have enhanced the vascular actions of isoflurane, a drug with known ability to reduce hypoxic pulmonary vasoconstriction (Lumb and Slinger 2015) and pulmonary arterial hypertension (Cheng and Edelist 1988; Ewalenko et al. 1997), potentially via the actions of nitric oxide and prostacyclin (Gambone et al. 1997).
Several limitations in our study should be considered along with our findings. First, inhalation exposure rather than the aspiration method is more reflective of real-world exposures. Though it has limitations, the aspiration method is useful in concentration-response studies by allowing delivery of known doses into the airways of experimental subjects. Second, we only examined selected time points following exposure including ventilatory responses immediately after exposure and cardiac ultrasound assessment one day later. We conducted these evaluations based on hypothesized physiological changes expected to occur after exposure, although this may have prevented complete documentation of the total scope of resulting health effects. Lastly, exposure to peat smoke PM does not fully capture effects that may result from more volatile constituents including gases of whole peat smoke.
In conclusion, this study provides evidence that exposure to peat smoke constituents can influence the regulation of left ventricular volumes and pulmonary artery hemodynamics, which could be linked to early pulmonary irritation responses. To fully establish a connection between airway irritation, altered autonomic function, and changes in cardiovascular physiology following peat smoke exposure, further study will be necessary. Though our findings are indicative of subclinical endpoints that may be transient in nature, they were still detectable without the use of experimental challenges. As such, diminished physiological feedback regulation of parameters like left ventricular volume could promote increased vulnerability to non-specific physiological stressors and exacerbation of disease. Given that environmental conditions may continue to favor the occurrence of peat fires and other wildland fires, an appreciation for the potential public health consequences related to development or worsening of cardiovascular diseases is pertinent.
Supplementary Material
AKNOWLEDGEMENTS
The authors have no conflicts of interest to disclose. We would like to thank Judy Richards for the serum and lavage fluid analysis, and Dr. Colette Miller and Dr. Steve Gavett for thorough reviews of the manuscript.
Footnotes
DISCLAIMER
The research presented in the article was reviewed and approved for publication by the National Health and Environmental Effects Research Laboratory at the U.S. Environmental Protection Agency. The contents of this manuscript do not necessarily reflect the views and policies of the agency nor does mention of trade names or commercial products signify endorsement or recommendation for use.
REFERENCES
- Abatzoglou JT, Williams AP. 2016. Impact of anthropogenic climate change on wildfire across western US forests. Proceedings of the National Academy of Sciences of the United States of America. 113(42):11770–11775. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argacha JF, Bourdrel T, van de Borne P. 2017. Ecology of the cardiovascular system: A focus on air-related environmental factors. Trends Cardiovasc Med. [DOI] [PubMed] [Google Scholar]
- Baker KR, Woody MC, Valin L, Szykman J, Yates EL, Iraci LT, Choi HD, Soja AJ, Koplitz SN, Zhou L et al. 2018. Photochemical model evaluation of 2013 California wild fire air quality impacts using surface, aircraft, and satellite data. Sci Total Environ. 637-638:1137–1149. eng. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- Carll AP, Haykal-Coates N, Winsett DW, Hazari MS, Ledbetter AD, Richards JH, Cascio WE, Costa DL, Farraj AK. 2015. Cardiomyopathy confers susceptibility to particulate matter-induced oxidative stress, vagal dominance, arrhythmia and pulmonary inflammation in heart failure-prone rats. Inhalation toxicology. 27(2):100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio WE. 2016. Proposed pathophysiologic framework to explain some excess cardiovascular death associated with ambient air particle pollution: Insights for public health translation. Biochimica et biophysica acta. 1860(12):2869–2879. [DOI] [PubMed] [Google Scholar]
- Chan EA, Buckley B, Farraj AK, Thompson LC. 2016. The heart as an extravascular target of endothelin-1 in particulate matter-induced cardiac dysfunction. Pharmacol Ther. 165:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DC, Edelist G. 1988. Isoflurane and primary pulmonary hypertension. Anaesthesia. 43(1):22–24. eng. [DOI] [PubMed] [Google Scholar]
- Cohen J 1988. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates. [Google Scholar]
- Deweirdt J, Quignard JF, Crobeddu B, Baeza-Squiban A, Sciare J, Courtois A, Lacomme S, Gontier E, Muller B, Savineau JP et al. 2017. Involvement of oxidative stress and calcium signaling in airborne particulate matter - induced damages in human pulmonary artery endothelial cells. Toxicology in vitro : an international journal published in association with BIBRA. 45(Pt 3):340–350. eng. [DOI] [PubMed] [Google Scholar]
- Ewalenko P, Brimioulle S, Delcroix M, Lejeune P, Naeije R. 1997. Comparison of the effects of isoflurane with those of propofol on pulmonary vascular impedance in experimental embolic pulmonary hypertension. British journal of anaesthesia. 79(5):625–630. eng. [DOI] [PubMed] [Google Scholar]
- Gambone LM, Murray PA, Flavahan NA. 1997. Isoflurane anesthesia attenuates endothelium-dependent pulmonary vasorelaxation by inhibiting the synergistic interaction between nitric oxide and prostacyclin. Anesthesiology. 86(4):936–944. eng. [DOI] [PubMed] [Google Scholar]
- Gaveau DL, Salim MA, Hergoualc'h K, Locatelli B, Sloan S, Wooster M, Marlier ME, Molidena E, Yaen H, DeFries R et al. 2014. Major atmospheric emissions from peat fires in Southeast Asia during non-drought years: evidence from the 2013 Sumatran fires. Scientific reports. 4:6112. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr MW, Youtz DJ, Eichenseer CM, Smith KE, Nelin TD, Cormet-Boyaka E, Wold LE. 2015. In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function. American journal of physiology Heart and circulatory physiology. 309(1):H53–62. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal A, Akram M, Del Monaco A, Smith K, Sim MR, Meyer M, Tonkin AM, Abramson MJ, Dennekamp M. 2015. Impact of Fine Particulate Matter (PM2.5) Exposure During Wildfires on Cardiovascular Health Outcomes. J Am Heart Assoc. 4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka H, Noguchi I, Putra EI, Yulianti N, Vadrevu K. 2014. Peat-fire-related air pollution in Central Kalimantan, Indonesia. Environmental pollution (Barking, Essex : 1987). 195:257–266. eng. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. 2011. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environmental health perspectives. 119(7):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, Lefler JS, Blagev DP, Korgenski EK, Torosyan N, Hansen GI et al. 2018. Short-term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. American journal of respiratory and critical care medicine. eng. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Fussell JC. 2017. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic Biol Med. 110:345–367. [DOI] [PubMed] [Google Scholar]
- Kim YH, Tong H, Daniels M, Boykin E, Krantz QT, McGee J, Hays M, Kovalcik K, Dye JA, Gilmour MI. 2014. Cardiopulmonary toxicity of peat wildfire particulate matter and the predictive utility of precision cut lung slices. Particle and fibre toxicology. 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Warren SH, Krantz QT, King C, Jaskot R, Preston WT, George BJ, Hays MD, Landis MS, Higuchi M et al. 2018. Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environmental health perspectives. 126(1):017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou YR, Lai CJ. 1994. Reflex changes in breathing pattern evoked by inhalation of wood smoke in rats. Journal of applied physiology (Bethesda, Md : 1985). 76(6):2333–2341. eng. [DOI] [PubMed] [Google Scholar]
- Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Ledbetter A, Walsh L, Farraj A, Hazari M. 2017. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol. 324:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis MS, Edgerton ES, White EM, Wentworth GR, Sullivan AP, Dillner AM. 2018. The impact of the 2016 Fort McMurray Horse River Wildfire on ambient air pollution levels in the Athabasca Oil Sands Region, Alberta, Canada. Sci Total Environ. 618:1665–1676. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance SG, Laurance WF. 2015. Peat fires: emissions likely to worsen. Nature. 527(7578):305. eng. [DOI] [PubMed] [Google Scholar]
- Lumb AB, Slinger P. 2015. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 122(4):932–946. eng. [DOI] [PubMed] [Google Scholar]
- McGuinn LA, Ward-Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, Schwartz J, Koutrakis P, Russell AG, Garcia V et al. 2017. Fine particulate matter and cardiovascular disease: Comparison of assessment methods for long-term exposure. Environ Res. 159:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen GD, Hougaard KS, Larsen ST, Hammer M, Wolkoff P, Clausen PA, Wilkins CK, Alarie Y. 1999. Acute airway effects of formaldehyde and ozone in BALB/c mice. Human & experimental toxicology. 18(6):400–409. eng. [DOI] [PubMed] [Google Scholar]
- Page SE, Hooijer A. 2016. In the line of fire: the peatlands of Southeast Asia. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 371(1696). eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CM, Hazari MS, Farraj AK. 2015. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc Toxicol. 15(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold AG, Stone SL, Cascio WE, Neas LM, Kilaru VJ, Carraway MS, Szykman JJ, Ising A, Cleve WE, Meredith JT et al. 2011. Peat bog wildfire smoke exposure in rural North Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environmental health perspectives. 119(10):1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT. 2016. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environmental health perspectives. 124(9):1334–1343. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RK, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Chen S, McGilvery CM, Hu S, Shaffer MSP, Bonvini SJ et al. 2018. Mechanistic link between diesel exhaust particles and respiratory reflexes. The Journal of allergy and clinical immunology. 141(3):1074–1084.e1079. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira V, van der Velden J. 2017. The Frank-Starling Law: a jigsaw of titin proportions. Biophysical reviews. 9(3):259–267. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. 2008. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. Journal of the American College of Cardiology. 52(9):719–726. eng. [DOI] [PubMed] [Google Scholar]
- Sommer N, Strielkov I, Pak O, Weissmann N. 2016. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. The European respiratory journal. 47(1):288–303. eng. [DOI] [PubMed] [Google Scholar]
- Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. 1995. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 26(6):357–366. [PubMed] [Google Scholar]
- Thompson LC, Ledbetter AD, Haykal-Coates N, Cascio WE, Hazari MS, Farraj AK. 2017. Acrolein Inhalation Alters Myocardial Synchrony and Performance at and Below Exposure Concentrations that Cause Ventilatory Responses. Cardiovasc Toxicol. 17(2):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinling MA, West JJ, Cascio WE, Kilaru V, Rappold AG. 2016. Repeating cardiopulmonary health effects in rural North Carolina population during a second large peat wildfire. Environ Health. 15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbancok D, Payne AJR, Webster RD. 2017. Regional transport, source apportionment and health impact of PM10 bound polycyclic aromatic hydrocarbons in Singapore's atmosphere. Environmental pollution (Barking, Essex : 1987). 229:984–993. eng. [DOI] [PubMed] [Google Scholar]
- Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. 2006. Warming and earlier spring increase western U.S. forest wildfire activity. Science (New York, NY). 313(5789):940–943. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


